1/2
2/2
https://www.intechopen.com/chapters/72928
https://web.archive.org/web/20231221010714/https://www.intechopen.com/chapters/72928
Written By Hilmiye Deniz Ertuğrul Uygun and Zihni Onur Uygun
Abstract
Sensor and biosensor technologies have shown rapid progress in recent years. These technologies use nanomaterials that have an important place in immobilization materials for recognition analyte molecules. Although fullerenes among these materials have attracted much attention in recent years, their number of studies is less than other carbon-based nanomaterials. Thanks to its completely closed structure and at least 30 double bonds, it can be modified from 30 points, which provides a great advantage. At these points, thanks to the ability to modify amine, thiol, carboxyl or metallic groups, modification residues can be created for all kinds of immobilization. According to the zero-dimensional nanomaterial class, fullerenes provide an extremely large surface area. Therefore, it provides more biological or non-biological recognition receptors immobilized on this surface area. Moreover, increasing the surface area with more recognition agent also increases the sensitivity. This is the most important parameter of sensor technologies, which is provided by fullerenes. In this book chapter, the development of fullerene-modified sensor and biosensor technologies are explained with examples, and fullerene modifications are given in figures as fullerene derivatives. Contribution was made in the method development stage by giving comparison of fullerene type sensor and biosensor systems.
1. Introduction
Carbon, which is the source of our lives, apart from our metabolic activities, attracts attention with its extraordinary structures created in nature. These structures are materials that are formed and discovered over time in the environment of high temperature and pressure [1]. The chemical properties of these materials are very different from those of inert carbon. Conductivity, strength, and catalytic properties are only a few of the carbon nanomaterials. By taking advantage of these features, the ability of today’s technology to further develop products or make R&D increases. This, together with costs, can facilitate the development of technology.
With the development of biotechnology and the interdisciplinary sciences over time, the use of nanoscale materials is increasing in biotechnological process developments. Nanomaterials created new opportunities especially in biotechnology, with their easy modification advantage, especially in the field of diagnosis, and they offered significant advantages over traditional diagnostic methods in terms of sensitivity and selectivity. Diagnosis is the most important step in terms of developing health technologies. The correct diagnosis brings with it the rightful treatment, the right prognosis, the well-being of the patient, and the decrease in health expenditures. The important parameters in the exact diagnosis are sensitivity and accuracy. These two terms can describe the technological power of the diagnostic systems. In the development of sensitivity and accurate measurement, nanomaterials have an important place in today’s technologies [2].
Among the various nanomaterials, carbon nanomaterials offer wide advantages due to their outstanding electrical, thermal, chemical, and mechanical properties [3]. Composite materials derived from carbon nanomaterials are used in energy storage and conversion, sensors, drug delivery, field emission, and nanoscale electronic components [4].
Depending on the purpose of use, carbon nanomaterials increase sensitivity by increasing surface area and conductivity especially in diagnostic systems. A promising sub-branch of diagnostic systems has made great progress in recent years, creating an important area in the development of point-of-care diagnostic tests. This area is especially developed on the fundamentals of sensor and biosensor technology. The technology consists of a recognition agent placed on a physicochemical transducer. In this simple system, electrodes, optic systems, or piezoelectric systems can be used as physicochemical transducers. Electrodes are physicochemical conductors that can detect electrochemical signals in a solution. On the other hand, optical sensors can detect light-matter interactions, and piezoelectric systems can perform specific and sensitive mass analysis. The recognition layer on these transducers plays a key role for biosensors and sensors. In biosensor systems, this recognition receptor is called biorecognition agent such as enzymes, antibodies, DNA, RNA, and other proteins that can be used as biorecognition elements [5]. As a result of the interaction of these biomolecules with the target molecule, catalytic or affinity-based biosensor systems can be developed. Otherwise, molecularly imprinted polymers, nanoparticles, and other polymers can be used as recognition agents in sensor systems instead of biological receptors [6]. Increasing the effectiveness of these recognition agents depends entirely on the properties of the immobilization/modification material used in the modification of the physicochemical transducer. Fortunately, nanomaterials can be used in sensor and biosensor systems in order to increase the power of the measurement system or to use it as recognition materials. These materials increase the surface area to obtain more sensitive signals and increase the possibility of interacting with more target molecules by binding more recognition agents to the surface. Technically, nanomaterial forms of inert metal/organic materials can be used as catalytic agents when they are in nano form.
In this book chapter, the production method, modification, and use of fullerene nanomaterials, which is a nanomaterial in the development of biosensor and sensor systems developed with biological or non-biological recognition agents, are described.
2. Fullerenes
2.1 Fullerene structure
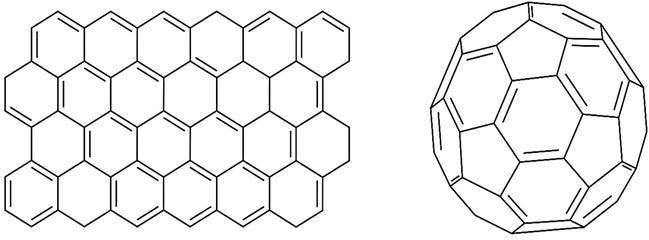
Production and applications of carbon-based nanomaterials have gained speed in recent years. Carbon nanomaterials that can be found in different conformations with Sp2 hybridization can be extremely useful (Figure 1). These nanomaterials include nanotubes, graphene, carbon nanoparticles, carbon fibers, and fullerenes [7]. Among these materials, fullerenes are nanomaterials that have gained speed in recent years due to their structure and unique properties.
Fullerenes are spherical carbon nanomaterial derivatives. This structure, unlike other carbon structures, consists of a closed form of pentagonal and hexagonal carbon structures fused together [1].
Graphene nanomaterials are called two-dimensional nanomaterials because they consist of only one layer, while fullerenes are classified as zero dimensional closed cage type nanomaterial. The spherical form of the fullerene nanomaterial gives this nanomaterial a large surface area [8, 9, 10]. This feature is a sought after feature for biosensors and sensor systems. The most advantageous feature of the double bonds formed by the carbon structure is that they can be modified as they can easily respond to chemical reactions. Most of the chemical reactions occurred by the nucleophilic attacks can form active sides on the fullerene sphere. With these modifications, fullerenes can be chemically modified. Chemical modification is important for biomolecule immobilization or surface modification. Fullerene in 60 carbons has the capacity to form 30 bonds due to its spherical structure and 30 double bonds. These bonds can be modified with different chemical agents to form fullerene derivatives.
2.2 Fullerene derivatives and chemical reactions
The most isomerically found types of fullerene derivatives are C60 and C70 fullerenes. These derivatives can only be dissolved in highly non-polar liquids such as toluene and benzene. Thanks to this hydrophobic property, it can be used as a drug carrier, intercalator or modification material in hydrophobic layers as lipid layers. In computer studies about fullerene, solubility attempts that were made in 75 different solvents were examined [11].
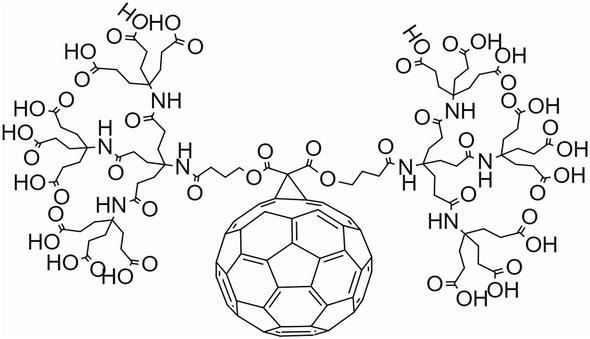
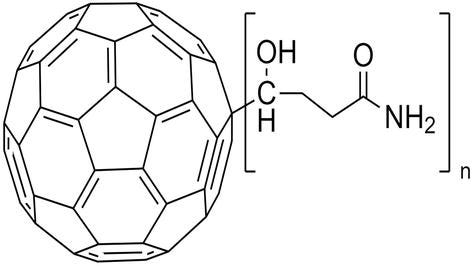
Although it is possible to dissolve in different solvents, surface modification of fullerenes for biosensor and sensor technology is the most effective way to use fullerene. The solubility in water can be achieved by being modified with polar groups. The method developed by Hirsch and colleagues, fullerene was modified by 18 carboxyl groups, gained solubility in water as 34 mg/mL at pH = 7.4 [12]. In these studies, a nucleophilic cyclopropanation procedure was performed; the protection was removed with the help of bis-(polyamide)-malonate dendrimer; and 18 carboxyl groups were created (Figure 2). With this modification, a material, which can be used as an advantage in water solubility for modification of the carboxyl group, especially for sensor and biosensor technology, is obtained. With the activation of EDC/NHS, 18 carboxyl groups can be made to bind the amino group or a fullerene nanomaterial containing carboxyl groups can be modified on an amino group-modified transducer.
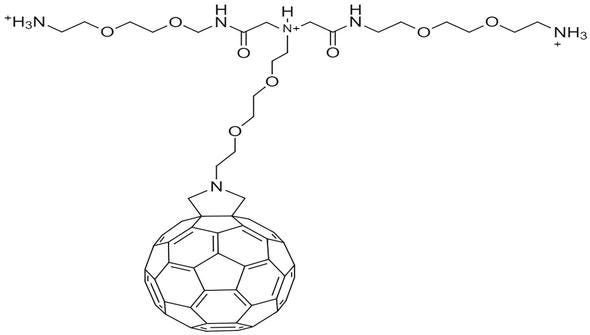
In another study, Cusan and his colleagues synthesized three ethylene glycol and three ammonium groups in poly charged fullerene-pyrrolidone derivatives [13]. In this study, which was carried out on two strategies, 2,2′-(ethylenedioxy)diethylamine was reacted by amino esterification with benzyl bromoacetate. Subsequently, the substance was interacted with carboxylate groups on fullerene to obtain fulleropyrrolidine in toluene. In this method, the authors have shown that purification is difficult and yield is low. For fullerene-PEG, in terms of biosensor and sensor technology, the fullerene derivative has two different arms with an amino group that can be used for modification by activating amino groups with glutaraldehyde a cross linker (Figure 3).
Fullerene modifications can be carried out entirely through the modification of the C = C bonds on fullerene. Fullerene modifications and derivatives of these modifications are seen in several studies. Accordingly, fullerene can be used to develop biosensors and sensors after being modified [14].
Apart from their use for modification material, another interesting feature of fullerene and fullerene-like materials is their photocatalytic advantage. The C60 shows a semiconductor-diode-like behavior and shows photo activity when illuminated at a value close to 1.4 eV [1]. This photocatalytic feature shows that it can be used as a photo catalyst in optical sensors and biosensors.
In conclusion, due to its catalytic properties, the use of fullerene is not only used as an immobilization material but also it is used in biosensor and sensor systems.
3. Fullerene-modified sensors and biosensors
Sensor and biosensor technology is an important start line in the development of miniature analyzers. This technology is divided into two classes depending on the interaction of the sensor and analyte molecule: if the analyte molecule is transformed on the sensor surface called as catalytic based and if the analyte interacts with the surface called affinity-based sensors and biosensors [5]. The measurement can be performed electrochemically, optically, and piezoelectrically. In electrochemical sensors, electrodes are used as transducers. Different types of electrodes can be used according to the modification and measurement principle. The electrodes can be gold, carbon, platinum, and their derivatives. It is desired to create a modification layer over self-assembly monolayer; thiol containing chemicals can be used for gold and gold derivative electrodes [15]. Carbon derivative electrodes can be used for polymer production and adsorption type studies [16]. Thus, measurement is carried out with electrodes modified with the immobilized recognition agent. If the recognition agent is a catalytic agent (enzyme or nanoparticle), the electroactive species are released as a result of the target molecule that is transformed by recognition receptor. The method of measurement may also vary depending on the type of these species. For example, an amperometric technique is used if an electroactive species is formed, or a potentiometric measurement method is used if an ion is formed. In addition, interaction-based measurement is desired without a reaction between the analyte molecule and the recognition receptor on the electrode surface (DNA, antibody, MIP, polymer, etc.), and impedimetric techniques can be used [15, 17, 18].
Optically designed sensor and biosensor systems use optic systems with optic sensitivity as transducers or surface plasmon resonance systems, which are a highly sensitive system that is used quite frequently today with laser canteen systems. The photons detected by the transducer with chemical reaction light sensing capability that occurs in optic systems can be converted into meaningful signals. In cantilever systems, measurement is performed by creating differences in the angle of the reflected laser signal as a result of the analyte molecule, which is attached to the surface of the cantilever, whose laser signal is reflected under a lever, changes the oscillation of the lever [19]. In surface plasmon resonance type sensors, the laser signal reflected behind the gold bit surface changes with the binding of the target molecule to the dielectric constant of this gold surface [20]. Here, too, the main purpose is the interaction between the analyte molecule and the recognizing receptor. If there is a catalytic effect, a photon sensing transducer, if there is an affinity-based measurement, cantilever or SPR sensor systems are used.
Apart from these two techniques, piezoelectric systems, which are mass detection systems, can be designed as affinity-based instead of catalytic in biosensor and sensor systems. The method principle is the analyte molecule interacts with piezoelectric crystals used as transducers can generate signals by changing the oscillation of the piezoelectric systems [21].
In conclusion, the interaction between the target molecule and the recognition receptor on sensor and biosensor systems can be measured with electrical, optic, and piezoelectric systems. The important point in these measurements is the characteristic of the modification layer on which the recognizing receptor is immobilized. As we mentioned above, the usage of nanomaterials in biosensor and sensor systems is to increase the surface area and electrical conductivity, to create more stable layer for immobilization, and to use nanomaterials catalytically by generating electroactive signals from the electrical characteristics of nanomaterials. With the advantages of these materials, more sensitive and selective sensor and biosensor systems can be developed.
3.1 Fullerene-modified sensors
As we mentioned above, sensor systems can use molecularly imprinted polymers, nanocomposites, and other polymeric or dendrimer materials as recognition agent, instead of a biomolecule from biological source, and measurement basis can be formed with it. In this section, examples of this type of sensor technologies are given with the use or modifications of fullerene nanoparticles.
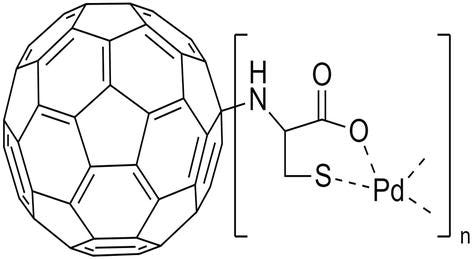
Zhong and colleagues performed the fullerene nanoparticles for catalytic activities, fullerenes were covalently modified with cysteine, and then palladium nanoparticles were added to this spherical structure to create Pd@Cys-C60 structure (Figure 4) to glucose detection without enzyme [22]. Firstly, the fullerene covalently bonded with the alpha amino group of cysteine on the nucleophilic attack basis in the medium containing the nanoparticles NaOH and EtOH. Then palladium cation was added, and Pd@Cys-C60 nanocomposite material was obtained. As is known, palladium is highly effective catalytic material in nano form. In this way, the researchers modified the glassy carbon electrode with this nanocomposite and developed a sensor system that can measure glucose between 2.5 μM and 1 mM, at the lowest detection limit of 1 μM. In selectivity studies, it gave 4.8% signal to substances such as fructose and ascorbic acid. As a result, sensitive and stable signals were obtained with fullerene, such as glucose sensors modified with previously made palladium nanoparticles [23].
Anusha et al. used fullerene nanomaterials with bimetallic nanoparticles to develop electrochemical sensors for vitamin D3 determination [24]. Copper and nickel metal nanoparticles were used in this study. Glassy carbon electrode was used as the working electrode, and this electrode was modified by dropping C60 in toluene. The C60 was further reduced in the solution KOH solution and then to the solutions containing CuNPs, NiNPs, and nanoparticles were deposited electrochemically on this electrode, respective electrodeposition steps. Modifying the electrode with these nanoparticles is due to their surface area enhancing and catalytic effects. VitD3 was measured by cyclic voltammetry by the electrochemical oxidation on NiNPs-CuNPs-C60-GCE. With the development of the method, 1.25–475 μM linearity and 0.0025 μM LOD values were achieved. As a result of the study, a more sensitive method was developed than similar methods [25, 26, 27, 28].
Saha and Das have developed a moisture sensor with C60 nanoparticles that immobilized on thick alumina layer [29]. In this study, they created nano-sized cavities on the sensor surface with the use of fullerene nanoparticles. With these gaps, they have improved the surface area and have developed a sensor that can detect even trace amount of moisture not in literature by fullerene advantage. The sensor was briefly prepared by heating a ceramic layer at 900° after being modified with fullerene, alumina, and polyvinyl alcohol, respectively. The sensor enables capacitive measurements to detect moisture at the ppm levels, in the range of 1–25 ppm. Supported with fullerene has the potential to be used successfully in the gas, oil, and food drying industries, due to the small pore sizes, a highly selective sensor has been developed for other volatile liquids.
Shetti and colleagues have developed a fullerene-modified GCE biosensor for electrochemical acyclovir (ACV) determination [30]. The GCE modification with fullerene was carried out according to the drying process on the previously mentioned GCE method. In this study, fullerene was dissolved in dichloromethane instead of toluene, dried by dropping on GCE and reduced in KOH. Afterwards, the electrode was immersed in ACV and the accumulation of ACV on the electrode surface was carried out electrochemically. ACV measurement was carried out with DPV, and real sample experiments were carried out by adding ACV to the real samples as spike. With this sensor, ACV linear measurement was achieved between 90 nM and 6 μM and 1.48 nM lower limit. The sensor gave more sensitive results compared to other ACV measurement methods by modification with fullerene [31, 32, 33, 34, 35]. Tartaric acid made the most interference on the sensor with 11.6%. In real sample experiments, a matrix effect of less than 3% is observed.
Ertuğrul Uygun and colleagues have developed an impedimetric sensor system modified with cortisol-imprinted polymers on fullerene for the determination of cortisol in saliva [36]. In this study, fullerene C60 was dispersed in dimethylformamide and dried on a carbon screen printed electrode. COOH groups were then formed on fullerene by oxidation process in 2 M H2SO4. Afterwards, acrylamide was polymerized with APS around cortisol via these COOH groups, and cortisol-imprinted polymers were synthesized on the electrode surface. After removing cortisol with acetic acid, the CE-C60-polyAcry sensor is ready for the determination of cortisol (Figure 5). In this study, which was performed in real saliva samples and compared with tandem mass spectrometry, the cortisol measurement was performed between 0.5 and 64 nM and the lowest detection limit of 0.14 nM was achieved. Modification of fullerene with carboxyl groups facilitated the synthesis of polymers and increased surface area. Sensor regression analysis compared to tandem mass spectrometry was found to be 0.9778.
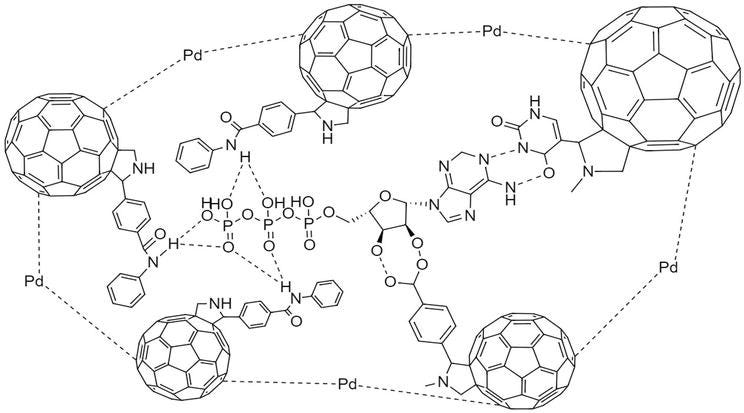
Another sensor study developed the preparation of molecularly imprinted polymers by making them more advantageous with fullerene for the determination of ATP by Sharma and his colleagues [37]. In this study, fullerene C60 nanomaterials were primarily modified with amide derivatives. After this modification, fullerene derivatives assembled around ATP by crosslinking them with Pd nanoparticles (Figure 6). The fullerene derivatives used herein are amide, carboxyl, and uracil functionalized fullerene nanomaterials. This sensor is characterized by complex measuring systems. Concentration determination was carried out capacitive, and characterization of the polymer layer was performed piezoelectrically and voltammetrically. The ATP measurement was carried out piezoelectrically. 0.062 to 1.0 mM linear ATP was measured capacitively, and the lowest detection limit was 0.31 mM.
3.2 Fullerene-modified biosensors
Biosensor systems use biorecognition agents from biological origin as the recognizing agent. These systems can also be catalytic and affinity-based just as mentioned before. Enzymes are used in catalytic biosensors. The important point here is a catalytic biosensor system can measure the electroactive species or mediators released by enzymatic reaction. In affinity-based biosensors, the basis of the measurement is based on the measurement of the interaction between the biorecognition receptor and the target molecule. These interactions are DNA-DNA, DNA-protein, antigen-antibody, or protein-ligand. In this section, fullerene-modified biosensor systems are discussed.
Pan and Shih fullerenes have developed a piezoelectric-based immunosensor system with C60 nanomaterials [38]. In this system, piezoelectric quartz crystals were modified by spin-coating the toluene solution containing polyvinyl chloride and fullerene onto these crystals. An adsorption type modification was carried out by adding anti-immunoglobulin G on this modification. In this biosensor, IgG determination reached the determination range between 0.0001 and 0.01 mg/mL and the lowest detection limit below 0.0001 mg/mL and has shown a very high selectivity.
Suresh and colleagues have developed another C60 modified immunosensor [39]. With this sensor, prostate specific antigen was determined, and the lower measurement limit of 0.002 ng/mL was reached. The biosensor can detect PSA in a measuring range from 0.005 to 20 ng/mL. GCE was used as the working electrode in the study. The C60 dissolved in toluene and dried on GCE. CuNPs were then deposited electrochemically on fullerene which was electrochemically reduced in NaOH. Then, the electrode was immersed in hydroquinone (HQ) solution and electrochemically bound to surface by the application of potential. After that, HQ-modified electrode was activated with EDC/NHS, and anti-PSA was covalently immobilized to this surface. PSA was determined by measuring the hydrogen peroxide reduction of HRP by forming a layer-by-layer on electrode by blocking the active ends exposed with BSA and the secondary antibody labeled with PSA and HRP, respectively. Electrochemical characterization was performed by CV and EIS. The measurement was carried out with the CV method by the principle of measuring the reduction of the HQ. The biosensor in serum showed a more sensitive result than similar studies with its fullerene modification, showing a 2% matrix effect [40, 41, 42, 43, 44, 45].
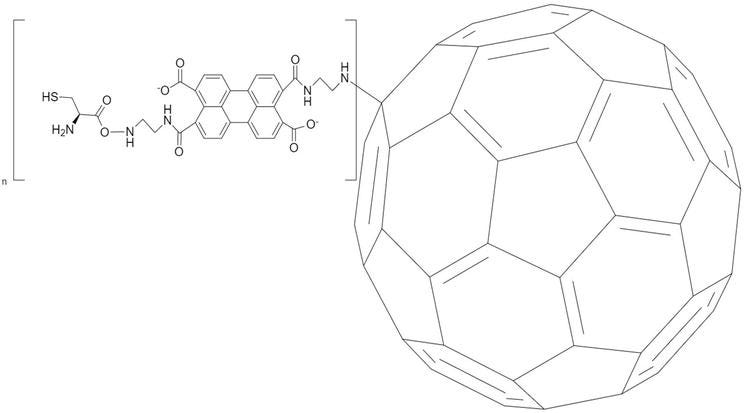
Zhou et al. performed an ultra-trace amount of miRNA-141 measurement in another fullerene-modified biosensor study [46]. In this study, a new method has been developed that provides a double signal increase. G-quadruplex, which is complementary with miRNA-141, was combined to form the DNA-RNA hybrid. The DNA fragment was then cut with a duplex-specific nuclease, and miRNA-141 was released to triggering the next step and measuring. The reason of the system’s ultra-sensitivity is that by C60-modified gold electrodes modified with amino and thiol groups. The fullerene dissolved in toluene, then passed to the water phase by removing toluene. PTC-NH2 was obtained by adding anhydrous ethylenediamine to the solution containing 3,4,9,10-perylenetetracarboxylic dianhydride (PTCDA) (Figure 7). PTC-NH2 was mixed with the fullerene in the aqueous phase to form amid groups on the fullerene. Afterwards, more active groups were formed by adding EDC/NHS on C60 by activated Cys. Gold electrode modification was carried out by forming the Au-SH bond of SH groups on the modified C60. Electrochemical characterization of the biosensor was carried out by EIS and CV, and differential pulse voltammetry (DPV) was used as the method of analysis. The target miRNA-141 was determined with this biosensor to measure between 0.1 pM and 100 nM, and the lowest detection limit of 7.78 fM was achieved. As a result of increasing the surface area with fullerene on developed biosensor, the researchers have reached a very low detection limit.
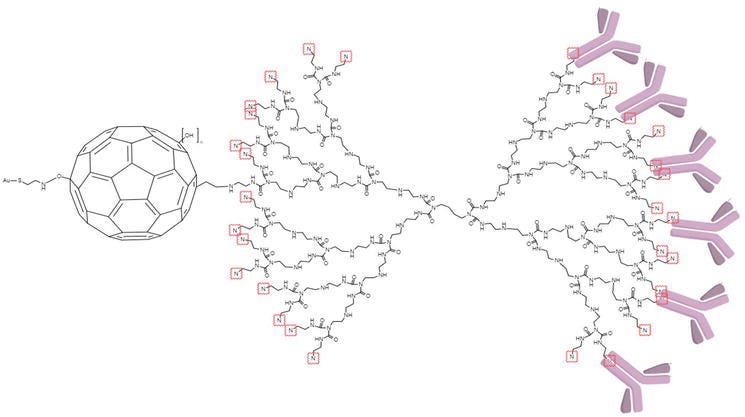
Uygun et al. have developed an impedimetric Fetuin-A biosensor, modified with Fullerene-PAMAM (G5) [15]. In this study, the gold electrode, as a working electrode, was first modified by forming self-assembly mono layer of 4-aminothiophenol (4-ATP). Then, activated by EDC/NHS poly-hydroxylated fullerene was firstly dropped to this surface. Au-4ATP-C60-OH was modified by PAMAM (G5) dropped to the surface and anti-Fetuin-A antibodies were dropped to the surface, respectively (Figure 8). The biosensor compared to ELISA provided linear measurement between 5 and 400 ng/mL and the lowest 1.44 ng/mL measurement opportunity. The fullerene-PAMAM modification has shown that the biosensor is more advantageous than ELISA due to its three-minute determination and high surface area.
Chuang et al. measured glucose with the piezoelectric system using C60 modified with glucose oxidase enzyme [47]. After the enzyme fullerene and glucose oxidase was incubated for 70 hours, it was immobilized on the piezoelectric crystal. In the sensor system where glucose was measured, LOD was found to be 39 μM. It is stated that in the system, which has a linear glucose measurement between 100 μM and 10 mM, glucose measurement can be made in real samples.
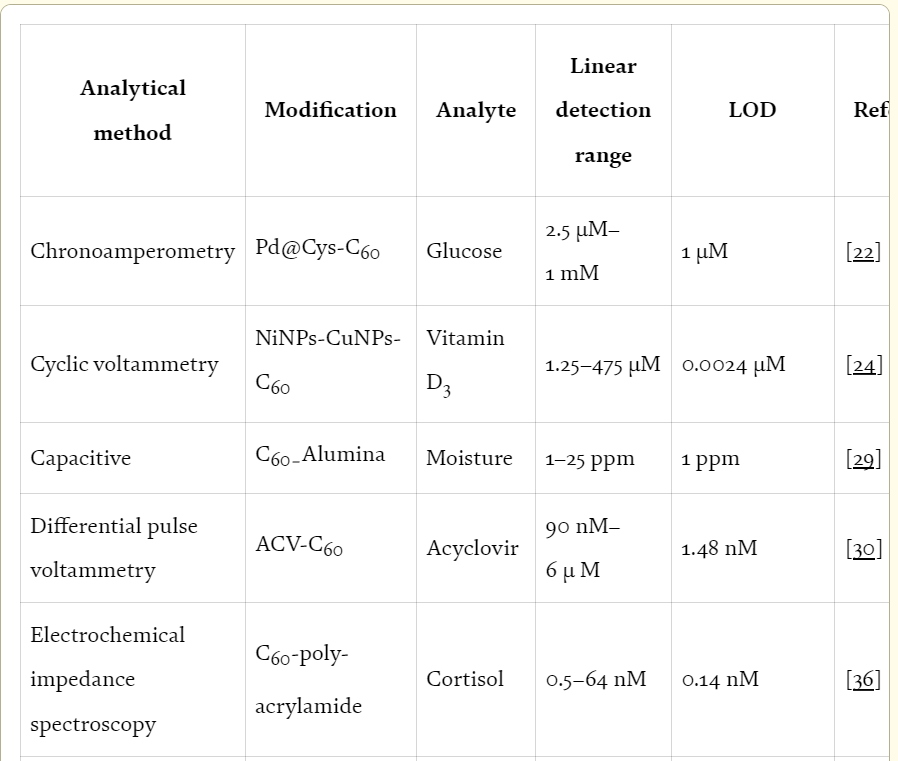
Sensor and biosensor studies have shown that the use of fullerene has been an advantage in these systems. It is summarized in Table 1 with the complete example.
Table 1.
Comparison of the fullerene-modified sensor and biosensor technology.
4. Conclusions
Nanomaterials are becoming more important and interesting day by day and their usage areas are increasing. In this book section where the sensor and biosensor systems modified with fullerene are explained, how the fuller is adapted to these systems and how it is modified is explained with examples. As a result, fullerene has successfully completed the task and contributed significantly to the development of these systems. The use of fullerene enabled the development of sensitive sensors by increasing the surface area. At the same time, its contribution to being an indestructible immobilization material and providing durability cannot be ignored.