Well, apparently these criminals don't want that people can check how toxic graphene/graphene oxide and their derivatives really are and compare them with the side effects of these toxic substances,
https://phmpt.org/wp-content/uploads/2021/11/5.3.6-postmarketing-experience.pdf
because the url to the pdf file given here
And here you can see what damage this material can cause....
has disappeared, but still stored in the WebArchive! So if you want to record something that is important, you should reproduce everything!
https://web.archive.org/web/20230102232356/https://eprints.bmsu.ac.ir/2499/1/Toxicity%20of%20carbon-based%20nanomaterials%20Reviewing%20recent%20reports%20in%20medical%20and%20biological%20systems.pdf
1. Introduction
With the advancement of nanotechnology over the past four decades, there is a growing rate for application of nanomaterials in different aspects of our life [1,2]. According to the collected information by the National Nanotechnology Initiative (NNI), the production and applications of nanoscale material are increasing remarkably every year [3]. Nanomaterials due to size scale and aspect ratio have numerous applications in different fields of human life including industry, medicine, pharmacy, biology, agriculture, cosmetic, and communication products [4,5]. Based on studies performed by Vance et al. there had been a 30-fold increase in nano-based products between 2011 and 2015 [6]. There are different types of nanomaterials that are applied in biology and medicine for different applications such as drug delivery, imaging, nanobiosensors, regenerative medicine, etc. [3,7,8]. Despite many advantages of the nanomaterials, toxicity effects are normally expectable. It can be originated from high dose usage by users, not being standard by producers, leaking into the environment, occupational exposure for workers, and many others [9–11]. There is a global aim to reduce these negative effects by developing some regulations for production, working with or using the nanomaterials or their end-used products [1,12,13]. In this manner, the first step is to know every possible toxic effect of each nanomaterial which has been performed through many experiments. It can be assessed in the environment or in living organisms such as in vitro and in vivo effects on the organism, tissue or cells [14,15]. For better understanding the nanobio interaction, researches have made a great effort to perform experiments and revealed unknown surprises every day in both in vivo and in vitro studies [16–18]. There are even some computer technology and simulation methods to investigate nanomaterial-biomolecules interactions [19]. Some new researches suggest that some important information regarding nano-bio interfaces such as biomolecular corona or even cell parameters such as type, sex, size, passage number, etc., should be taken into account [20–22]. Today, we have a better understanding of the nanotoxicity based on nano-bio interaction knowledge. The aim of this article is to review the recent progress and reports regarding the toxicity of the carbon-based nanomaterials (CNMs) and structures of widely used nanomaterials.
2. Nanomaterials
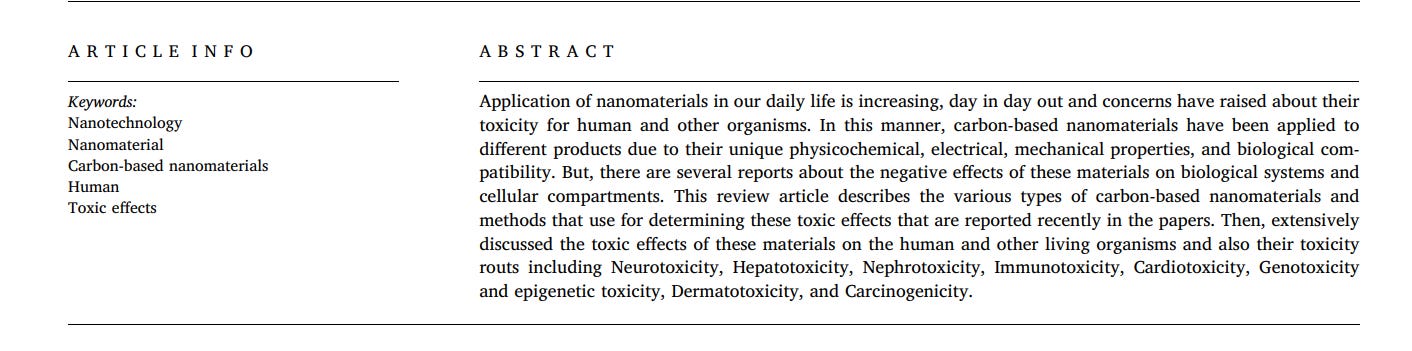
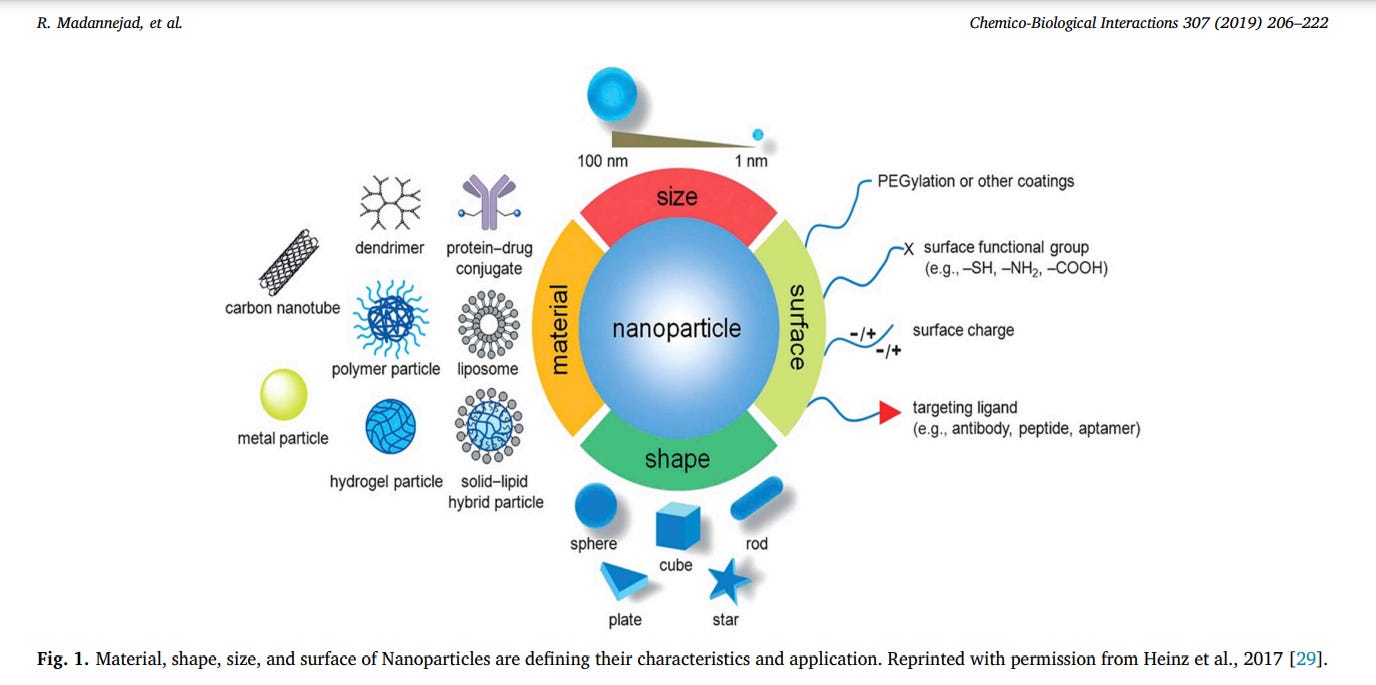
Nanoparticles size is less than 100 nm in length or height or diameter. In this dimension, because of quantum effects, substance exhibits different characteristics than the micro sizes. According to these new characteristics, scientists have discovered many applications for different types of nanomaterials so far [23,24]. Based on their shape, nanomaterials can be divided into some classes such as 0D (spherical forms such as gold nanoparticles or nano fullerene), 1D (tube and wire forms such as carbon nanotube or gold nanowire), 2D (sheet and plate forms such as graphene or graphene oxide), and 3D (any 3D structures such as gold nano cube and DNA tetrahedral structures) [25,26]. The physicochemical characteristics of nanomaterials, including their chemical composition, shape, size, stability, functionalization, charge, porosity and hydrophobicity/hydrophilicity, agglomeration or aggregation, mainly affect their interactions with biological molecules [17,27,28]. In Fig. 1, the most important parameters of nanoparticles are summarized as an illustration. In addition, in Fig. 2, the impact of nanoparticle characteristics in their toxicity is represented as a schematic.
3. Carbon-based nanoparticles
There are several types of CNMs including fullerene, graphite, carbon black, graphene, graphene oxide, reduced graphene oxide, and carbon nanotubes.
3.1. Fullerenes
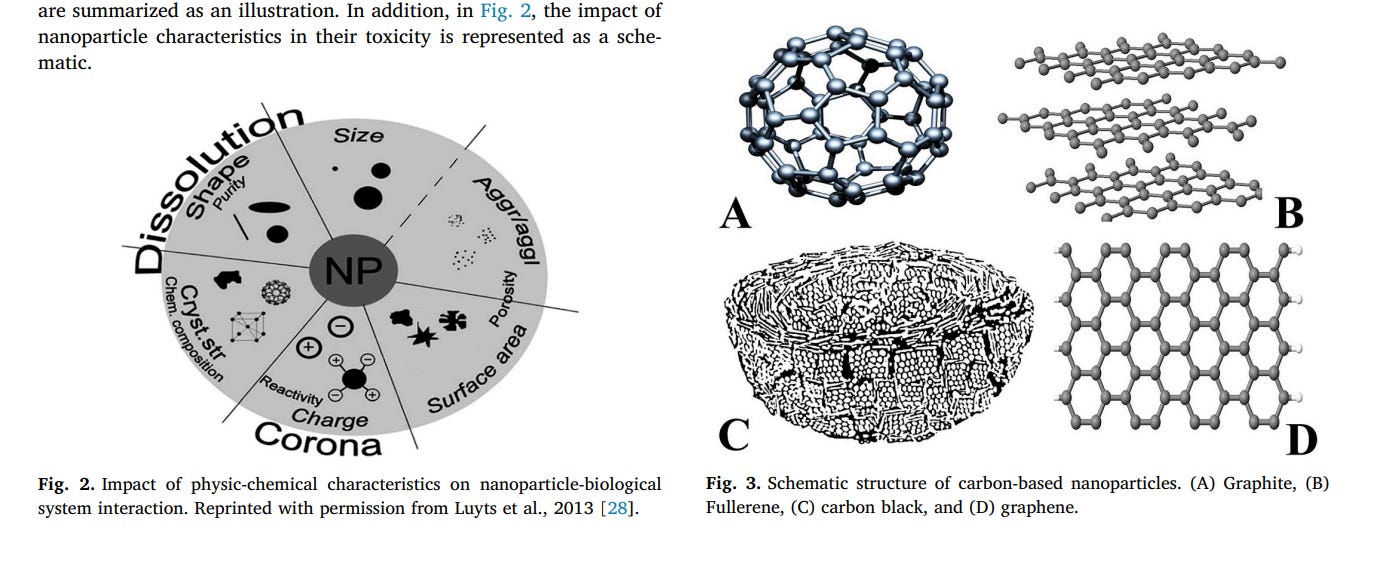
Fullerene (Fig. 3A) is the third allotropic form of carbon [30]. Fullerenes were discovered in the mid-1980s by Harold. W. Kroto, Robert F. Curl and Richard E. Smalley who were later awarded the Nobel Prize for chemistry in 1996 [30,31]. The fullerene name has been taken off the architect Buckminster Fuller that was renowned for designing and constructing geodesic domes [32]. C60 is the most common fullerene, also called buckminsterfullerene [30,31]. It is the smallest fullerene molecule that consists of 60 carbon atoms, arranged as 20 hexagons and 12 pentagons to form a spherical structure similar to a
soccer ball with a diameter of approximately 1 nm [30,32]. There are three forms of fullerenes containing spheres, ellipsoids or tubules [33]. The chemical formula of spherical fullerenes is Cn, that “n” indicates the number of carbon atoms in the molecule structure [34]. Fullerene molecules come in several forms and sizes ranging from 30 to 3000 carbon atoms [35]. Fullerenes with the less than 300 carbon atoms, or endohedral fullerenes, are generally named as buckyballs [30]. In contrast, fullerenes with more than 300 carbon atoms are commonly known as giant fullerenes that they exhibit in different forms including single-shelled or multi-shelled carbon structures, onions, and nanotubes [30] (see Fig. 4).
The first technique used for fullerenes production was based on laser ablation of graphite targets in a He gas [32]. This method produces low amounts of fullerenes, thus, mostly used to research purposes [32]. In 1990, Kratschmer et al. for the first time presented a method for producing large amounts (several grams per day) of fullerenes [32,36]. They employed an alternating current (AC) arc discharge between high purity graphite rods in 100–200 Torr of helium (He) or Argon (Ar) gas [32]. The combustion technique commonly used to synthesize fullerenes that are able to produce soot with a high yield of these compounds [34]. The occurrence of combustion is done by mixing toluene, acetylene, or benzene with oxygen [34]. Fullerenes are resistant to heat, pressure, and radiation [37]. In addition, due to their unique electron-hybridization pattern of sp2 bonds, are highly configurable [37]. The fullerenes can be used as organic photovoltaics (OPV) [30]. Also, they showed antibacterial activity against different bacteria, such as E. coli, Streptococcus spp. And Salmonella [38]. In addition, fullerenes have received widespread attention as gene and drug delivery vehicles [35].
3.2. Graphite
Graphite (Fig. 3B) is a naturally occurring crystalline form of pure carbon. There are two main categorizations of graphite, natural and synthetic. Natural graphite is subdivided into three types including amorphous, flake, and high crystalline. It is formed through contact or regional metamorphism of sedimentary organic matter. Synthetic graphite can be created from coke and pitch. In Graphite, each carbon atom connects to three other carbon atoms via C–C bonds (120°) in the x-y plane. The C–C bond length and the spacing between carbon layers are 1.42 Å and 3.35 Å, respectively. Graphite is a good electricity conductor because it has delocalized electrons that are free for moving within the graphite structure. It has both metallic and nonmetallic features [39–42]. In addition, graphite has an industry application as a refractory material due to its high thermal stability. When a graphite crystal is converted to nanoparticles, edges which surround the graphite particles appeared. The presence of open edges around the peripheral region lead to unique properties in nano-graphite compounds that are distinct from their closed surface counterparts, such as fullerenes and carbon nanotubes. Graphite nanoparticles are basically obtained via the natural flaky graphite exfoliation and represent a range of platelet thickness from less than 0.34–100 nm [42,43].
3.3. Carbon black
The carbon sphere (CS) term refers to a spherical form of carbon that can reveal in a solid, hollow or core-shell morphology [44]. These materials have been given many names including carbon nanospheres, carbon balls, carbon blacks, carbon microbeads, mesoporous microbeads, etc. [45]. Carbon black (CB) (Fig. 3C) is one of the oldest forms of the spherical carbons [44]. Since historical documentation manifests that it was used to write letters in ancient Egypt and China [44]. CB has a nanostructure that is composited of carbon with a low amount of hydrogen and oxygen [46]. The concentric organization of the graphite layers in each particle is a specific property of CB that these layers are found to be more pronounced in the region close to the particle surface [34]. This nanomaterial displays a set of notable characterizes containing the high surface area, high thermal and electrical conductivity, and very low cost [47]. It was broadly used in several applications from scientific to industrial levels, owing to their unique physical, chemical, and electrical properties [48,49]. The most total manufactured CB is consumed in the rubber industry [49]. Furthermore, it is used in other fields such as inks, paints, plastics, coatings, electrostatic discharge compounds, ultraviolet light absorption applications, and as a chemical reagent [48,49]. Frequently, the pure CB particle Printex 90 has been employed as a model particle for the primary toxicology studies to represent percent carbonaceous particles in air pollution assay [49,50]. Also, CB is known as an alternative material for modifications surface in designing electrochemical devices, especially sensors and biosensors [47]. The mass production of CB is typically performed by thermal decomposition of natural gaseous, decomposition of acetylene, decomposition of ethylene in a plasma arc, and incomplete combustion of oil droplets under controlled conditions [34,48]. In contrast, laser ablation of graphite and laser pyrolysis of acetylene methods are used for laboratory scale production of CB [34]. These methods produce carbon blanks with unique physical and chemical features [34].
3.4. Graphene
Graphene (Fig. 3D) is a two-dimensional (2D) allotropic form of sp2 - hybridized carbon, described by some as the ‘mother’ of all graphitic carbon materials due to its discovery associated with the identification a broad range of novel two-dimensional materials such as carbon nanotubes (‘rolled up’ graphene sheets), and graphite (stacked graphene sheets connected together by powerful van der Waals's forces) [51,52]. It was first exfoliated mechanically from graphite in 2004 by Novoselov, Geim, and co-workers [53–55]. In recent years, graphene has been produced using different methods including mechanical exfoliation, chemical exfoliation, chemical synthesis, thermal chemical vapor deposition (CVD), plasma enhanced chemical vapor deposition (PECVD), thermal decomposition of SiC, un-zipping nanotube, and microwave synthesis [53,56,57]. Graphene has many exceptional characteristics, such as extremely high mechanical rigidity, flexibility, thermal stability and conductivity, large theoretical specific surface area, high Young's modulus, optical transparency, charge carrier mobility and a work function which can be regulated via electrostatic or chemical doping [31,58,59]. Moreover, its good electric features are essentially different from the properties of three-dimensional (3D) materials [31]. Due to these suitable properties, graphene and its derivatives have various applications in different fields of medical and physical sciences [60]. The numerous of the potential applications of graphene are in field emission (FE) displays, field effect transistors (FET), transparent conductive films, batteries, desalination of water, metal detection and its removal, biosensors, and tissue engineering [56,59,60].
3.5. Graphene oxide
Graphene oxide (GO) is the most famous derivative of graphene [61]. It is defined as a thin carbon nanostructure with various polar oxygen-containing functional groups such as hydroxyl (OH), carbonyl (C]O) carboxyl (C–O–OH), and alkoxy (C–O–C) on both the basal planes and edges [62–64]. The presence of these oxygenated groups lead to dispersibility and solubility of GO in water and other organic solvents and can strongly improve its mechanical, electronic, and electrochemical properties [62,65]. GO-based material nanocomposites have several applications in different fields including wastewater treatment especially in heavy metal removal, electronic devices (supercapacitor and lithium-ion batteries), self-cleaning technology, catalyst, biomedical and sensor design [66,67]. This nanomaterial has also shown expanding applications in biomedicine including drug delivery, optical imaging, and chemical sensors [8,68]. It is not a natural product and results in a non-stoichiometric molecule [63]. GO can be produced in both dry and wet medium [63]. In a dry medium, the GO is synthesized using oxidation reaction of graphene under ultrahigh vacuum condition and treating with ozone [63]. While in wet synthesis, graphite is typically employed as a graphene source, due to its natural abundance and inexpensive cost [63]. Brodie, Staudenmaier, and Hummers are three major methods used for preparing GO [63]. These synthetic methods associated with some limitations including lab safety ascribed to hazardous reagents, use of sodium nitrate or potassium chlorate in Brodie or Staudenmaier methods results explosive, and also sodium nitrate or fuming nitric acid in Hummers' method introduce heteroatoms or faults in the GO structure [63]. Therefore, for enhancing the overall yield and quality of the produced GO a number of new methods were derived from these basic methods, for instance, the Tour method, free-water exfoliation method, and Sun method [63]. The interpretation of physical-chemical characteristics of GO is not often easy because the majority of organic molecules in this material are carbons and oxygens [63]. The most regular techniques that use to identify the GO are FTIR, Raman spectrum, UV–vis spectra, X-ray diffraction (XRD), transmission electron microscope (TEM) and scanning electron microscope (SEM), atomic force microscopy (AFM), thermogravimetric analysis (TGA), and differential scanning calorimetry (DSC) [63].
3.6. Reduced graphene oxide
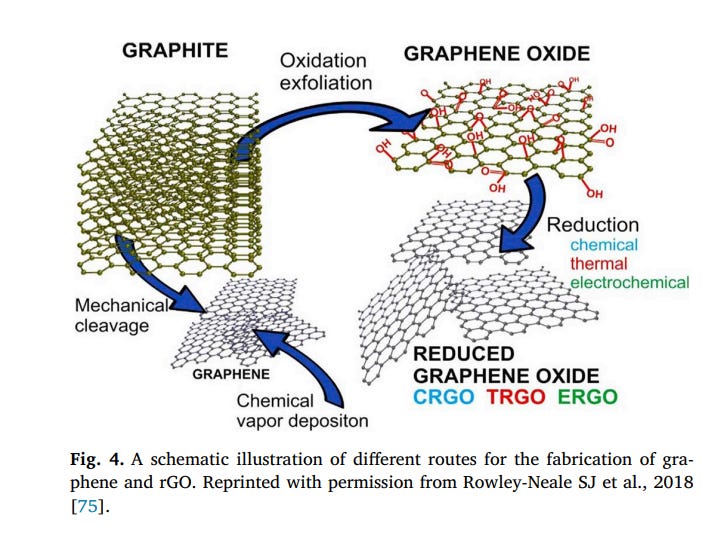
Reduced graphene oxide (rGO) is considered as one kind of chemically derived graphene that it is also named as functionalized graphene, chemically modified graphene, or chemically converted graphene. It usually produced from the oxidation/exfoliation of graphite to GO and after its reduction to graphene in several ways such as thermal, chemical and electrochemical [69,70] (Fig. 4). In thermal treatment, rGO is obtained by functionalization for modifying the edges [71]. In this method, GO is reduced spontaneous in the presence of other chemicals [71]. For chemical reduction GO and production rGO, hydrazine hydrate, hydroquinone, dimethylhydrazine, sodium borohydride, HI and Fe and Zn powder have been employed [72]. There are various criteria for determining changes that occur in GO before and after the reduction process including visual characteristics, electrical conductivity, carbon to oxygen atomic ratio (C/O ratio) [73]. After reduction, the C/O ratio is remarkably increased (12:1 in most cases) [73]. The C/O ratio is usually calculated via elemental analysis and Xray photoelectron spectrometry (XPS) analysis [73,74].
3.7. Carbon nanotubes
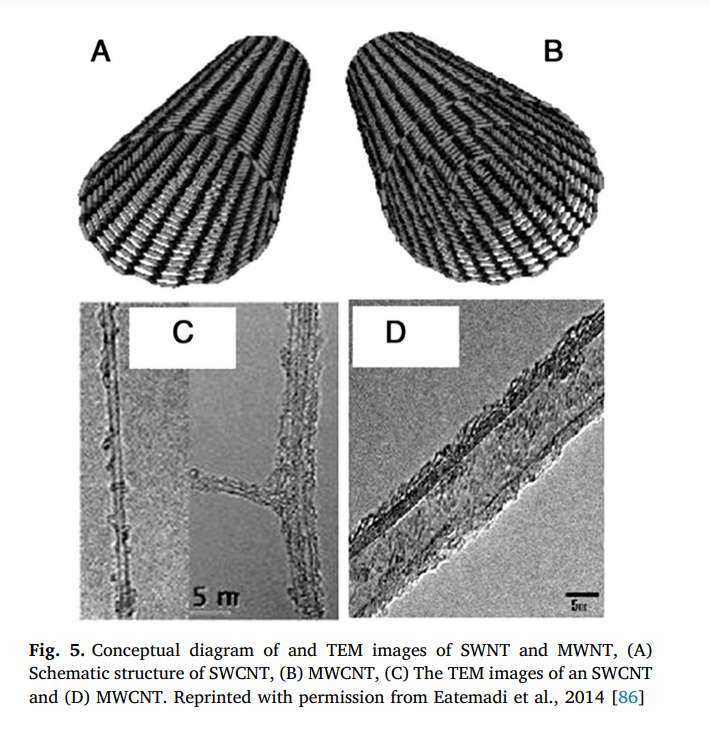
Carbon nanotubes (CNTs) are one of the carbon allotropes that belong to the fullerene family [76]. They can be described as a carbon atoms sheet rolled up into a nanoscale-tube that they were discovered by Iijima in 1991 [76]. Nanotubes generally have a large length-todiameter ratio close to 1000, so they can be known as nearly one-dimensional structures [77]. Conventional methods for producing CNTs are laser ablation, arc discharge, electrolysis, synthesis from bulk polymers, mechanothermal, furnace, (CVD), and (PECVD) [77–79]. The liquid pyrolysis and bottom-up organic are other less common techniques that can be used for CNT synthesis [80]. Every synthesis method has its own advantages and application in various fields. Among the methods mentioned CVD due to simplicity, controllable mechanism, high efficiency and appropriate for mass fabricating; is the most marvelous way for the synthesis of CNTs [77]. With CVD methods, the orientation, length, diameter, alignment, density, and purity of CNTs can be controlled exactly [80,81]. Due to the produced CNTs usually contain a large number of impurities and the average purity is about 5–10%, purification of CNTs must be performed [82,83]. For this work, air oxidation, acid refluxing, surfactant aided sonication, filtration, and annealing steps are done [82,84]. CNTs are the strongest materials ever known by humankind [77]. They are 100 times more resistant and 6 times lighter than steel. Despite their high rigidity, CNTs display extraordinary flexibility. Depending on the arrangement of graphite cylinders, CNTs are categorized into two types: single-walled carbon nanotube (SWNTs) and multiwalled carbon nanotubes (MWNTs) (Fig. 5). indications a diagram of SWNT and MWNT [85].
3.8. Single-walled carbon nanotubes (SWNTs)
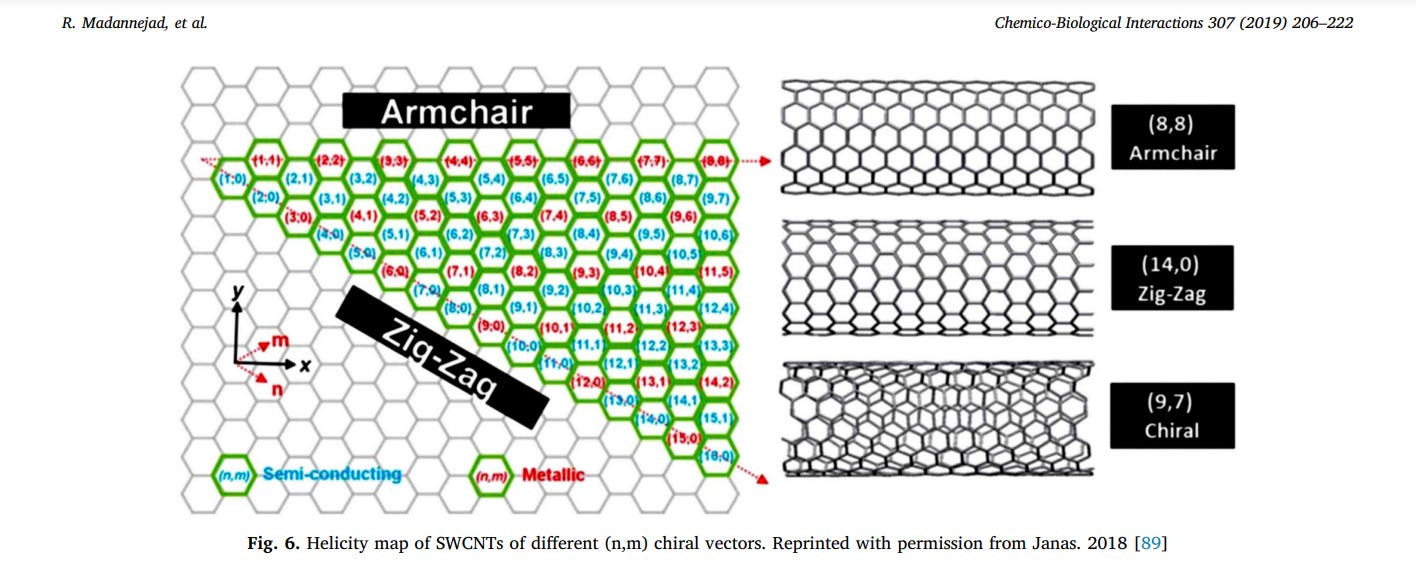
SWCNTs are mono-cylindrical carbon layers, made by rolling one sheet of graphene and represented via a pair of indices (n, m) called the chiral vector. These integers indicate the number of unit vectors along two directions in the graphene honeycomb crystal lattice [79,87]. Based on m and n values, SWNT can be further categorized into three classes including armchair (m = n) with α = 30°, have metallic character, zigzag (m = 0) for which α = 0°, can be either semi-metallic or semiconducting, depending on the specific diameter, and chiral (other values of m and n) with chiral angles intermediate between 0 and 30°as illustrated in (Fig. 6) [87–89] (see Fig. 7). SWCNTs are highly absorbent materials with powerful optical absorption in the near-infrared range; therefore they are used in the photo-thermal field and photoacoustic imaging [90]. For separating produced SWCNTs, electrophoresis, chromatography, centrifugation, selective solubilization, and selective reaction techniques are used [91].
3.9. Multi-walled carbon nanotubes
The Russian Doll model and Parchment model are two models that can be used to describe the structures of MWCNTs [79]. In the Russian Doll model, graphite sheets are arranged in concentric cylinders while in the Parchment model, a single sheet of graphite is rolled in around itself, like a rolled newspaper [79]. The interlayer distance in MWCNTs is calculated approximately 3.3 Å [79]. MWCNTs are commonly hydrophobic, so they are not easily dispersed in water [92]. For biological and biomedical applications, the lack of solubility of MWCNTs is the main technical barrier [93]. To overcome this problem and improve other biological properties such as biocompatibility, material compatibility, and cellular responsiveness, the MWCNTs surface can be chemically modified to present specific moieties (e.g., functional groups, molecules, and polymers) [93].
3.10. Magnetic carbon-based nanoparticles
Magnetic nanoparticles (MNPs) are a class of nanoparticles with magnetic properties [94]. Various metals can be used for synthesizing MNPs [94]. The most important benefit of using magnetic fields is their non-invasive nature, which makes them safe for biological structures [95]. In order to improve biocompatibility, organic and inorganic polymers such as RGD peptides, dextran, fibronectin, and different types of carbon-based nanoparticles can be used to coat the magnetic core [94]. By so performing, the useful features of both these compounds and MNPs lead to high functionalizing ability and targetable carriers [95]. It has been reported that the surface functionalization of CNTs with metallic nanoparticles lead to preparing the ideal and powerful nanohybrids [96]. This nanocomposite structures successfully employed in various fields such as biomedical imaging, fuel cells, gas sensors, data storage devices, supercapacitor, catalysis, and magnetic hyperthermia [96]. On the other hand, the presence hollow cavity in CNTs structure that can be filled with a variety of metals such as gold (Au), silver (Ag), copper (Cu), tin (Sn), iron (Fe), cobalt (Co), and nickel (Ni) and used as nanoantennas or microscopic probes [96]. In fact, the magnetic CNTs have opened a new route in the nanotechnology and its applications in
different fields, especially biomedicine [96]. Therefore, many researchers are focused on finding new methods for functionalization of CNTs (i.e., with magnetic or superparamagnetic nanoparticles) or in filling their cavity with magnetic molecules for designing and constructing versatile systems [96].
4. Nano-toxicology
While progress in nanotechnology is important and useful, but the hazards and negative effects of using nanotechnology must be assayed along with its advantages [97] (Fig. 7). Nanotoxicology is defined as a branch of bionanoscience that evaluate the toxic effects of nanoscale materials/particles on living organisms and other biological systems [3,98]. The behavior and toxicity of nanomaterial are related to their physical and chemical features such particle size, structure, shape, surface area, surface charge, solubility, catalytic activity, surface coatings, and active groups on the surface [5,99]. The small size of NPs allows them to pass through cell membranes and other biological barriers into living organisms causing cell damage. Also, the decrease in the size of nano-materials associated with a significant increase in particles surface area. Hence more chemical components can attach to the surface of nanomaterials and their reactivity and toxic effects are consequently increased. In 2012, Kim et al. evaluated the size-dependent cellular toxicity of silver nanoparticles (AgNPs) (10, 50, and 100 nm) on MC3T3-E1 and PC12 cell lines. Their results showed that the smallest sized AgNPs (10 nm) had a greater ability to induce apoptosis in the MC3T3- E1 cells than the other sized (50 and 100 nm) of AgNPs [100]. Koike et al. investigated chemical and biological oxidative effects of CB nanoparticles with mean aerodynamic diameters of 14, 56, and 95 nm in in vitro. They found that CBNPs with 14 nm size in comparison with larger sizes (56, and 95 nm) have a higher oxidative capacity and stimulate a more remarkable level of oxidative damage in alveolar epithelial cells [101]. Morphology, shape, and structure are other factors which play an important role in NP toxicity [5]. Generally, nanomaterials appear in different shapes and structures such as particles, tubes, fibers, spheres, rods, cubes, truncated triangles, wires, and films that effect on NP kinetics and their transport in the environment [5,102]. In 2015, Srikanth et al. assayed the cytotoxicity of four types of CNMs (carbon nanowire (CNW), (CNTs), graphene and fullerene) on L929 mouse fibroblast cancerous cells by MTT Assay. According to the SEM image, the CNWs have the perfect wire structure without any burrs. While graphene has a powdery appearance. The TEM image displays different layers of graphene nanoparticles that this morphology is flake-like and therefore has a high aspect ratio. Also, the SEM image obtained of CNT's shows that this nanoparticle has a high aspect ratio but considered one dimensional. Moreover, fullerene shows a truncated icosahedron structure. It can be seen that the four types of nanomaterials have completely different morphologies. The average cytotoxicity of CNW, CNT, graphene, and fullerene was evaluated in the days 3, 5, 7 and 10. Based on morphology, concentration and contact duration, graphene was the most toxic material with average toxicity of 52.24%, followed by CNTs, fullerene, and CNW. Obtained data represent that the differences in the toxicity levels are related to different structural arrangements and aspect ratio [103]. Chi Chuang et al. studied the surface area role and oxidative potential of CB in oxidative stress and DNA damage. Their results indicated that there is a positive relationship between CBNPs surface area and its oxidative potential. Also, they found that CB with smaller size and larger surface area demonstrate higher potencies for oxidative stress and DNA damage in rats [104]. The production of reactive oxygen species (ROS) is one of the most important toxicity mechanisms of NPs that lead to oxidative stress, inflammation, lipid peroxidation and result in damages to the proteins, cell membrane and DNA [105]. Oxidative stress is defined as an imbalance between the ROS production and the biological system's ability to detoxify the reactive intermediates (superoxide radical anions and hydroxyl radicals) that may be as a result an enhanced production of ROS, a decline in the cell's defense mechanisms, or a combination of both [5]. Shvedova et al. evaluated the adverse effects of SWCNT on human epidermal keratinocytes (HaCaT) using electron spin resonance (ESR) spin trapping. In this study, exposure of HaCaT cells to SWCNT accelerated oxidative stress. Furthermore, ultrastructural and morphological changes in cultured skin cells followed by CNTs exposure were observed [106,107].
5. Methods for evaluating the toxicity of carbon-based nanomaterials
Growing applications of nanomaterials is leading to increased exposure to nanomaterials, whether intentional or unintended, the
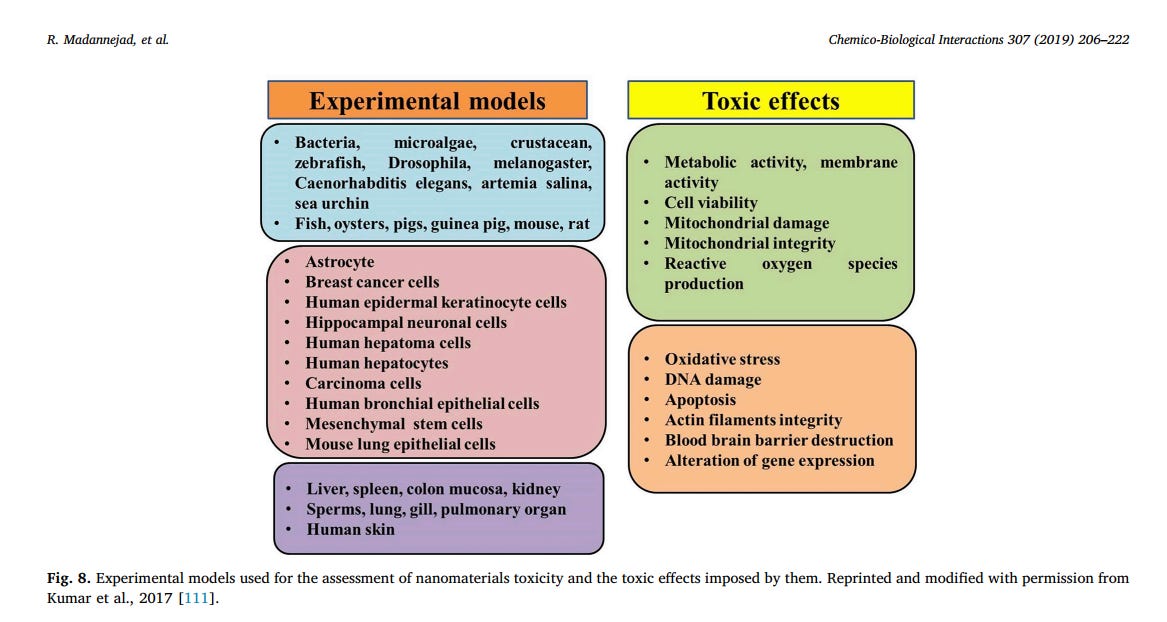
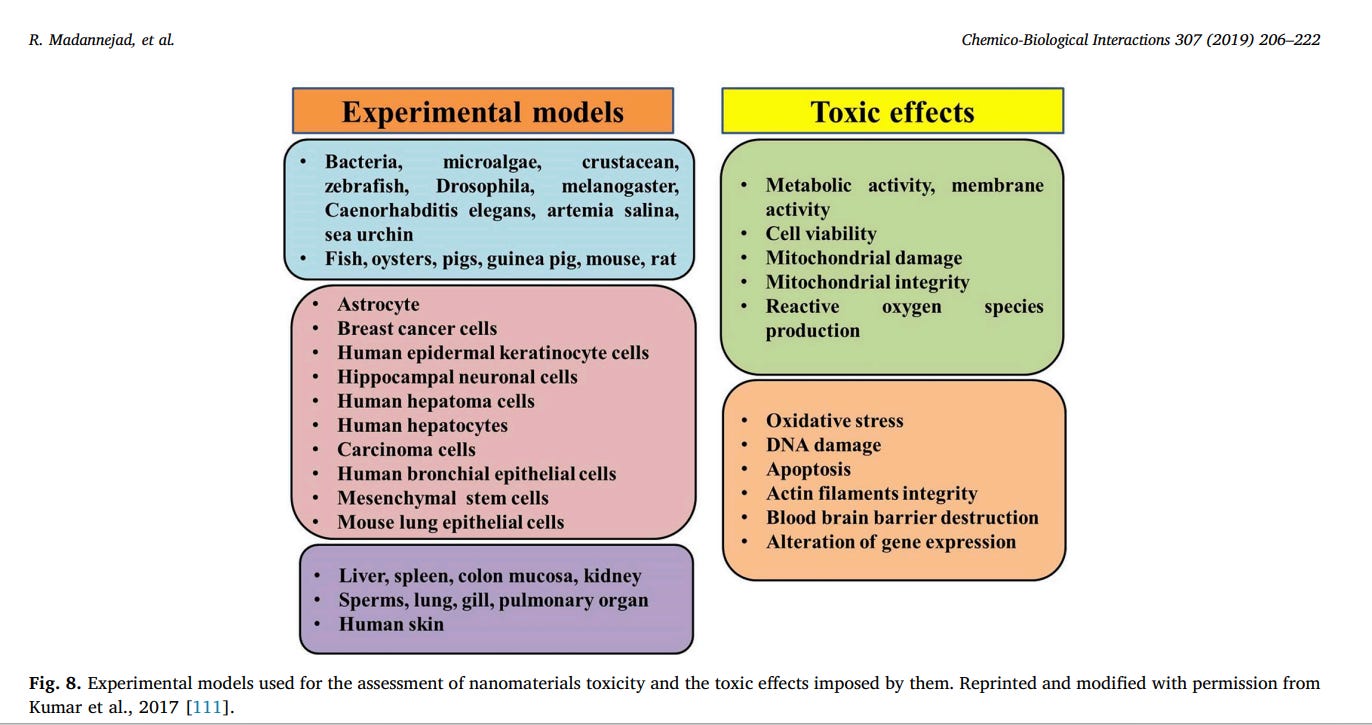
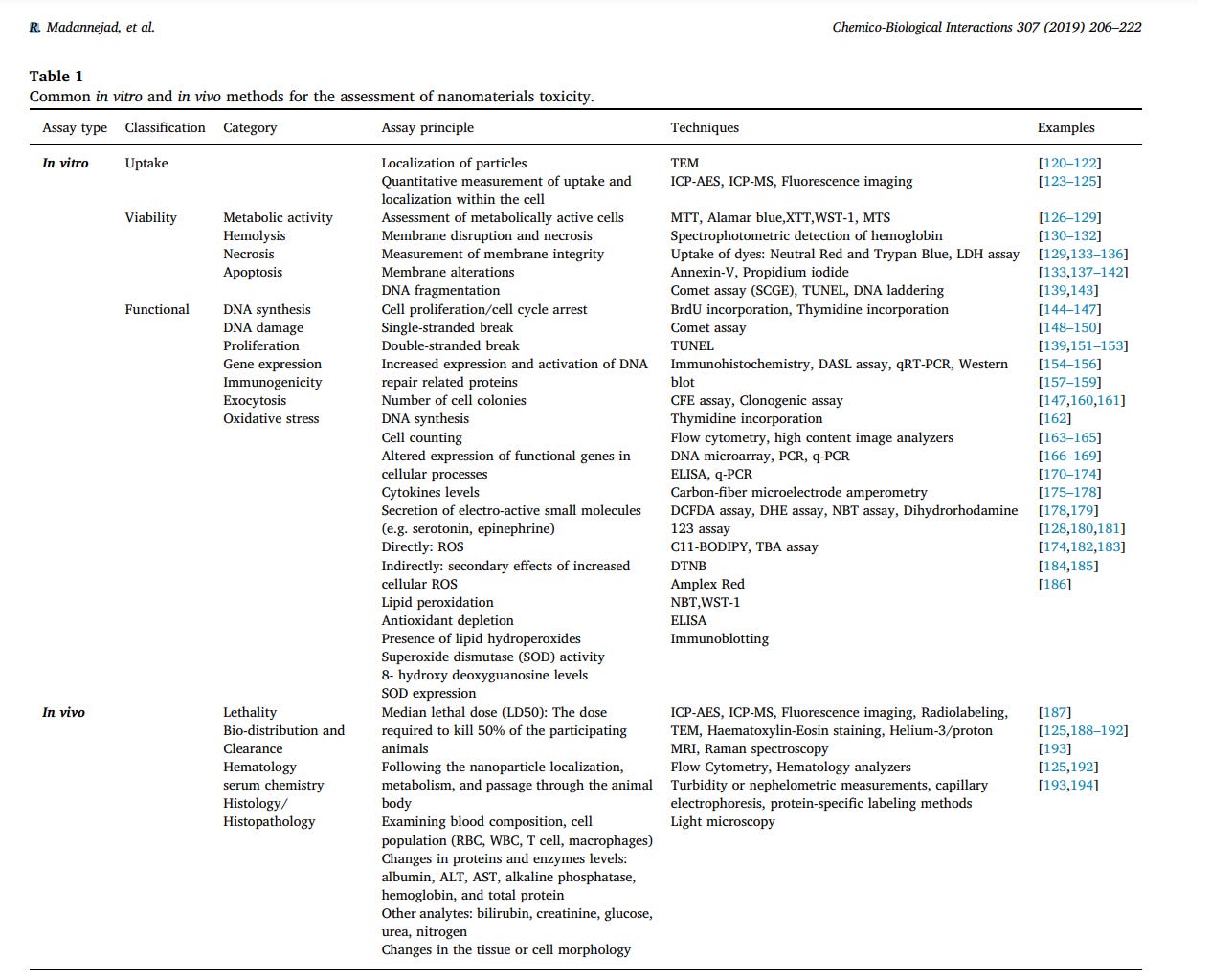
development of reliable toxicity screening tools is of crucial importance [8,108–110]. The common toxicity screening assays can be categorized as in-vitro and in-vivo assays. Fig. 8 presents the experimental models used for toxicity assessment of nanomaterials and the toxic effects induced following exposure to them. In vitro assessment methods have been more considered in recent studies due to the advantages like lower cost, faster procedure and minimum ethical concerns [111]. In vitro screening assays can be subdivided into three general categories: Uptake analysis, Functional assays and viability assays. Functional assays are defined as those that evaluate the effects of nanoparticles on various cellular processes, however, viability assays solely assess the induction of cell death [112]. The most commonly used in vitro methods and some examples regarding the application of these methods for the assessment of CNMs toxicity are summarized in Table 1. Indeed, cell-nanomaterial interactions should reflect all of the possible cell processing routes and physiological reactions to nanomaterials in vivo. However, prediction of in vivo toxicity using the results from in vitro screening techniques might be controversial due to different in vitro exposure conditions such as higher concentrations and exposure time as compared to in vivo cellular and environmental conditions [113].
In spite of various advantages and common application of these methods, CNMs have shown controversial results in some assays [114]. According to Herzog et al., these conflicting results may be due to the adsorptive nature of some of the CNMs [115]. The interactions between CNTs and the dyes used in some assays including 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), 2-(4-iodophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium (WST-1) and Red and Alamar Blue™ have led to false positive results [114,115]. Applying MTT assay for toxicity assessment of other CNMs such as graphene and GO has also resulted in false positive signals due to the spontaneous reduction of MTT by these CNMs [116,117]. Studies have revealed that ROS production assays can be affected by some nanomaterials including CB, producing ROS in cell-free systems and, therefore, leading to false positives [108]. Furthermore, selection of an appropriate cell line for in vitro experiments has been a critical concern, since even cell lines derived from a common cell source may exhibit controversial responses to a given stimulus. In a study by Magrez et al., the assessment of MWCNTs cytotoxicity in three different human lung tumor cells (H596, H446, and Calu-1) revealed that H596 cells had the highest sensitivity and resulted in more reproducible results [118]. Another major concern with in vitro studies is that the secondary culture of a cell line may no longer represent the original cell phenotypes, leading to confounding and unexpected results [108]. In vivo studies, in contrast to in vitro assays, provide more accurate and relevant information with a wider range of parameters including distribution, metabolism, and elimination and provide the opportunity for assessment of long-term chronic effects [114,119]. Toxicity of nanoparticles in in vivo models is determined based on dose, exposure conditions, exposure duration and route of administration [111]. In vivo experiments for assessment of nanotoxicity mainly fall into 6 categories including studies on LD50 (median lethal dose), analysis of blood serum chemistry, cell population, tissue morphology, histology and overall nanoparticle bio-distribution and clearance (Table 1). Most in vivo studies are concerned with different issues like nanoparticle choice and characterization, selection of the model system, exposure route, dose determination and proper selection of the target organ, tissue or cell type for histological and hematological analysis [113]. In vivo studies on nanoparticles cytotoxicity, unlike other compounds, may result in a wide variety of conflicting results [119]. These challenges mostly arise from the complexity associated with dose determination, optimization of nanoparticles dispersion and their interaction with the other biological structures following administration. The concentration of administered nanoparticles should mimic the actual dose that humans or animals are exposed to, however, determining the actual quantity of existing nanoparticles has been controversial due to special characteristics of nanoparticles including size and quantity. Meanwhile, exceeding a certain dose in in vivo administrations increases the risk of agglomeration. The relatively high surface area of nanomaterials has made them prone to agglomeration at physiological pH and salt concentration. Therefore, choosing an appropriate isotonic and non-toxic dispersant and optimization of dispersing conditions according to the route of administration remains challenging [119]. Furthermore, nanomaterials exposed to in vivo conditions would instantly encounter a broad range of surface-active molecules, different cell types, matrices and inflammatory conditions [108]. Interaction of these substances with different bio-structures may result in conformational changes in protein folding and alterations in biological functions and different signaling pathways [119]. Taken together, the multidisciplinary nature of nanotechnology has led to a variety of innovations, presenting significant challenges in the interpretation of cell and tissue toxicity [108]. Therefore, development and validation of toxicity assessment methods for engineered nanoparticles will be a crucial concern in the future [108,115]. Table instruction: TEM: Transmission electron microscopy; ICP-AES: Inductively coupled plasma atomic emission spectroscopy; ICP-MS: Inductively coupled plasma mass spectrometry; MTT: 3-(4,5-Dimethyl2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide; XTT: 2,3-bis-(2- methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide; WST1: Water soluble tetrazolium-1; MTS: 3-(4,5-dimethylthiazol-2-yl)-5-(3- carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium inner salt; LDH: Lactate dehydrogenase; SCGE: Single-cell gel electrophoresis; TUNEL: Terminal deoxynucleotidyl transferase dUTP nick end labeling; DASL: DNA-mediated annealing, extension, selection and ligation; ROS: Reactive oxygen species; CFE: Colony forming efficiency; ELISA: Enzyme Linked Immunosorbent assay; DCFDA: 2′,7′- Dichlorodihydrofluorescein diacetate; DHE: Dihydroethidium; NBT: nitroblue tetrazolium; BODIPY: Boron dipyrromethene; TBA: thiobarbituric acid; DTNB: 5,5′-dithiobis-2-nitrobenzoic acid; MRI: Magnetic resonance imaging; RBC: Red blood cell; WBC: White blood cell; ALT: Alanine aminotransferase, AST: Aspartate transaminase.
5.1. Toxic effects of carbon-based nanoparticles: in vitro and in vivo assessments
The emergence and rapid development of nanotechnology have led to numerous investigations on the environmental and health effects of nanomaterials [195]. In recent years, CNMs have been widely investigated for medical applications including drug delivery [196], electrochemical sensors [197], imaging systems [198], regenerative medicine [199,200], and cancer diagnosis and therapy [97,201,202]. Nanoparticles have been reported to accumulate in several organs including the heart, brain, kidneys, spleen, liver, and lung following inhalation, intravenous injection, and ingestion [177]. This section intends to review the cytotoxic effects of most studied CNMs in vitro and in vivo.
5.2. Neurotoxicity
Any adverse effect on the structure, chemistry or function of the nervous system, induced by a chemical or physical factor, can be referred to as neurotoxicity [203]. In contrast to conventional pharmacological therapies which display difficulties reaching to the central nervous system (CNS), some nanoparticles are able to pass the bloodbrain barrier (BBB). The BBB is a unique feature of the human body, maintaining brain homeostasis, regulating the transport of compounds and preventing toxic substances from reaching the brain. This feature has made some conventional therapies ineffective, therefore the development of effective and novel approaches for the treatment of CNS disorders remains a serious challenge [204]. Some nanoparticles, are capable of crossing the BBB and accumulating in several CNS areas due to their unique small size (< 1 μm) and high surface area. Accordingly, nanoparticles can be applied as a potential pharmacological carrier in the diagnosis, monitoring, and treatment of CNS related diseases [205]. However, their application as nanocarrier requires precise investigation, due to their possible adverse effects on CNS. Several in vitro and in vivo studies have demonstrated the neurotoxic effects of some nanoparticles inducing neuroinflammation and cognitive impairment [204]. Among various carbon nanomaterials, functionalized CNTs (fCNTs) have been shown to translocate into different subcellular compartments including brain cells [206]. Visalli et al. determined the neurotoxicity and neuroinflammation of pristine and functionalized multi-walled carbon nanotube (fMWCNTs, i.e. MWCNTCOOH) in differentiated SH-SY5 cells as in vitro model resembling neuroblastoma cells. They found that MWCNTs induced DNA damage and overproduction of reactive oxygen species in neuronal cells leading to pro-inflammatory response. The neuronal inflammation was confirmed by the increased levels of TNFα, IL-1β, IL-6 and cytokine levels [168]. In another study by Bussy et al. the cytotoxic impact of four different chemically functionalized MWCNTs (pristine MWNTs, carboxylated MWNTs, amino-functionalized oxidized MWNTs, and aminofunctionalized MWNTs) on glial and primary neuronal cells isolated from fetal rat frontal cortex (FCO) and Striatum (ST) was determined. The results of mLDH assay showed a significant decrease in the viability of ST-derived mixed glial cells (non-neuronal cells) following exposure to all types of MWCNTs (5–100 μgml−1), except for pristine MWCNT with the concentration of 5 μgml−1 which induced no toxicity following 24 h of incubation. In contrast, the mixed glial cells isolated from FCO brain region were not affected by MWCNTs at all concentrations from 5 to 100 μgml−1. Furthermore, the exposure of neurons isolated from both FCO and ST regions to the mentioned fMWCNTs at doses up to 100 μgml−1 did not induce any deleterious effect. Microglial cells are capable of maintaining brain homeostasis and protecting it against injury via engulfing foreign particles. Bussy et al. demonstrated that the uptake of CNTs, except for pristine MWCNT, was markedly higher in microglial cells compared to astrocytes. Taken together, their results indicated that the lower viability of ST-derived glial cells in comparison to FCO-derived cells has been due to a higher population of microglial cells in this region [206]. Recent studies have shown that GO is capable of entering animal cells via clathrin-mediated endocytosis leading to cytotoxicity [207]. Furthermore, GO has been reported to cross the BBB due to its amphiphilic properties which make it similar to the biological membranes [195]. In 2017 Hu et al. demonstrated the neurotoxic effects of GO nanosheets on the offspring of zebrafish through parental exposure. Thus, parental zebrafish were exposed to an aqueous environment containing 0.01–1 μgL-1 GO nanosheets. Their results exhibited no evidence of neurotoxicity in parental zebrafish. However, in contrast, the offspring showed numerous neurotoxic responses to GO including loss of dopaminergic neurons, decrease in acetyl cholinesterase activity, endoplasmic reticulum damage, autophagy, ubiquitin down-regulation and increased β-galactosidase activity (as an early biomarker of cellular senescence). Meanwhile, at the metabolic level, the failure of carbohydrate and fatty acid metabolism was positively proportional to the loss of dopaminergic neurons [195]. Onoda et al. determined dose-dependent effects of carbon-black nanoparticle (CB-NP), administered to pregnant ICR mice, on brain astrocytes of the offspring. These authors found that maternal exposure to CB-NP led to a dose-dependent rise in the expression of the glial fibrillary acidic protein (GFAP) and aquaporin-4 (Aqp-4) in cerebral cortex and parenchyma region around blood vessels, respectively. Meanwhile, changes in the expression levels of GFAP and Aqp-4 in the offspring following maternal CB-NP exposure were similar to the obtained results from mice of more advanced age. In addition, comprehensive alterations in expression levels of cortical mRNAs associated with proliferation, angiogenesis, chemotaxis, cell migration, and growth factor production were observed in the offspring after maternal CB-NP exposure. Their work revealed that maternal exposure to CB-NP induced astrocyte activation, leading to reactive astrogliosis and increased risk of age-related neurodegenerative disease [208].
5.3. Hepatotoxicity
Hepatic sinusoid and kupffer cells, as main structures for metabolism and detoxification, are prone to drug toxicity and deposition of nanoparticles [209]. The source of about 75% of hepatic blood is from the gastrointestinal viscera and spleen through the portal vein which brings drugs absorbed by the gut to the liver [207]. The liver accumulates 30–99% of administered nanoparticles from the bloodstream, leading to increased toxicity of hepatic cells. In 2017, Ahmadi et al. determined the hepatocytotoxicity of polyethylene glycol (PEG)-labeled SWCNTs and Tween-functionalized SWCNT (Tween-SWCNT) at doses of 75 and 150 μg/mouse in BALB/c mice after intravenous injection. Their results from the assessment of oxidative stress biomarkers and biochemical serum factors including malondealdehyde (MDA), glutathione (GSH) and liver enzymes alanine aminotransferase (ALT) and aspartate transaminase (AST) did not show any significant difference in the treated groups as compared to the controls. However, some histological injuries were observed in both groups especially in the mice exposed to Tween-SWCNT. The results of proteomics and Western blot analysis revealed that intravenous administration of PEG- and TweenSWCNT induced a significant increase in the expression of some liver proteins with antioxidant and detoxifying activities [210]. Some of these proteins include: Trap-1, protecting cells against oxygen-free-radical apoptosis and oxidative stress, Prx-6 protein, preventing lipid peroxidation, TPx, playing a protective role by detoxifying hydrogen peroxide and inhibiting lipid peroxidation, Rgn, preventing cell proliferation and apoptotic cell death, Gnmt, reducing the expression of oncogenes and INMT which its overexpression is involved in xenobiotic detoxification [210]. This proteomic approach can provide beneficial information on the mechanisms of toxicity and detoxification and the complex molecular changes within cells and tissues in response to different nanoparticles including CNMs [211–213]. The cellular detoxification pathways, such as anti-apoptosis and antioxidant defense pathways, have been reported to be activated in response to nanoparticle administration, reducing the oxidative stress-mediated cytotoxicity [211]. According to Shvedova et al. the surface functionalization of SWCNTs leads to a lower oxidative stress-dependent cytotoxicity [212]. In another study, Adedara et al. [214] demonstrated that repeated, short-term and intra-peritoneal administration of purified carboxylated MWCNTs induces hepatic oxidative damage in pubertal rats by disturbing antioxidant defense systems, promotion of cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS) levels and generation of pro-inflammatory cytokines. Their results indicated that liver damage in pubertal rats exposed to MWCNTs was accompanied by significant increase in serum activities of ALT [5], AST [207], gammaglutamyl transferase (GGT) and alkaline phosphatase [215] as compared with control. Meanwhile, they found that MWCNTs led to an increase in the activity of hepatic glutathione peroxidase (GPx) and increased levels of H2O2 and lipid peroxidation (LPO), indicating the GPx failure to reduce oxidative damage in the liver of treated rats. In a study conducted by Lin et al. the hepatotoxic effects of SWCNTs were investigated following intra-tracheal (IT) injection in male Wistar rats throughout a 15-day period. Biochemical analysis of plasma from the SWCNT-treated animals revealed a significant change in alkaline phosphatase, total cholesterol, and total protein. Meanwhile, the 1 H nuclear magnetic resonance (NMR) spectra of blood plasma and liver tissue extracts showed a significant alteration in the concentrations of glutamate, creatine, lactate, glucose, trimethylamine oxide, choline, HDL and VLDL as probable biomarkers of hepatotoxicity. Taken together, their results from clinical chemistry and metabolomics approaches comprehensibly indicated liver damage and disruption of energy, amino acid, and fat metabolism after exposure to SWCNT [216]. Earlier in 2009, Ji et al. reported the effect of intravenous injection of MWCNTs at doses of 10 and 60 mg kg−1 to Kunming mice. Their results showed that exposure to MWCNTs in a period of 15 and 60 days altered the expression of cytochrome P450, a cytochrome involved in drug metabolism, and therefore induced hepatotoxicity [217]. It is worth noting that other types of CNMs including GO [215–218], CB [219,220], and fullerene [221,222] have shown toxic effects on liver and are capable of altering its gene and protein expression.
5.4. Nephrotoxicity
Any adverse effect of substances on renal function is defined as nephrotoxicity. A nephron, the basic structural and functional unit of the kidney, is fundamentally involved in primary functions of the kidney including filtration of waste from the blood, maintenance of the body fluid balance, maintenance of blood pH and bone health, promotion of red blood cell production via hormonal functions and regulation of blood pressure. Loss of cells along any part of the nephron can induce kidney damage and renal failure [223]. CNTs are materials hardly dispersed in different solvents, leading to immunogenicity in the body and, in turn, causing significant limitations to their different applications [224,225]. Non-covalent functionalization of CNTs with surfactants, peptides, polymers, nucleic acids, and oligomers or introduction of moieties on the tube external surface can drastically improve their solubility [224]. A study on the cytotoxicity of polyethylene glycol-modified CNTs on human embryonic kidney cells (HEK-293T) showed that cell survival decreased gradually after exposure to increasing concentrations of PEG-CNTs. Exposure to PEG-CNTs at doses less than 100 μgml−1 did not induce toxicity in HEK-293T, however, at concentrations ranging from 100 to 200 μg/ml mild toxicity was observed [225]. In an earlier study, Reddy et al. reported that MWCNTs of two different sizes (60–80 nm and 90–150 nm) induced kidney toxicity in a dose-dependent manner. Exposure of HEK cells to MWCNTs with sizes in the range of 60–80 nm resulted in significantly higher cytotoxicity and oxidative stress as compared to the 90–150 nm nanotubes [177]. In another study, the impact of different types of MWCNTs, administered through intravenous injection to healthy mice, on the histology of different tissues including kidney was examined. Their results revealed that the higher degree of ammonium (NH3 +) modification on the surface of nanotubes resulted in less accumulation in the tissues. In addition, the histological analysis of kidney slides 24 h post-administration did not show any damage to glomerular physiology and no accumulation was observed for all types of studied MWCNTs including pristine, diethylentriaminepentaacetic- (DTPA) and
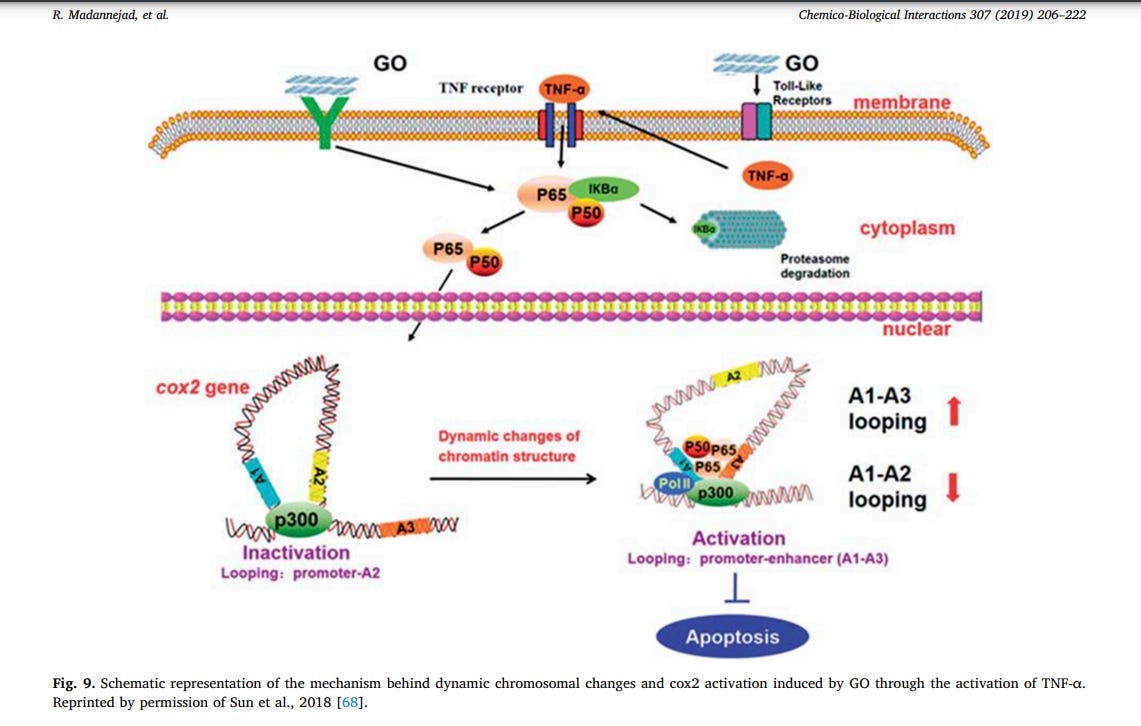
ammonium-functionalized nanotubes [226]. The increasing human exposure to GO has raised concerns over its potential adverse effects and cytotoxicity [68]. Different studies have reported the GO-mediated activation of multiple signaling pathways related to autophagy and necrosis in different types of cells [65,227–229]. Epigenetic regulatory mechanisms including histone modification, DNA methylation, chromosome remodeling, and small RNA regulation have been reported to induce nanotoxicity [68,230]. Dynamic chromatin organization, a crucial epigenetic regulatory mechanism, is capable of regulating gene expression levels and genome homeostasis. In a study by Sun et al., the nanotoxic effects of dynamic chromatin structural changes generated by GO was evaluated in in-vitro kidney cells (HEK-293T). The investigators demonstrated that GOtreatment dynamically changed the chromatin architecture at the cox2 locus, a mediator of inflammation, via activation of TNF-α–NF–κB signaling pathway. This process was associated with the activation of cox2 and induction of apoptosis via epigenetic mechanisms. Cox2, a key enzyme catalyzing prostaglandin production in response to different stimuli, regulates various biological reactions including proliferation, apoptosis, cell death and inflammation. The investigators speculated that the epigenetic effects of GO might have occurred through GO-cell membrane interactions, leading to an increase in the secretion of TNFα. The high levels of TNF-α leads to the formation of p56-p300 complex, changing the higher-order chromatin structure at the cox2 locus via binding to its promoter and enhancer regions (Fig. 9). Their results indicated that pristine and aminated GO (GO-NH2) significantly increased the expression of cox2, however polyacrylic acid-modified GO (PAA-GO) induced little effects on cox2 expression. Using chromosome conformation capture (3C) assay, it was found that PAA-GO did not significantly enhance dynamic chromatin interactions and, therefore, cox2 protein expression, leading to weaker inflammatory response [68]. These results are in consistence with previous studies [231–233], suggesting that GO surface modification can improve its biocompatibility [68].
5.5. Immunotoxicity
The immune system, a regulated network of cells, organs and molecules, recognizes and eliminates foreign particles [234]. The novel physicochemical properties of engineered nanomaterials (ENM) have made them a potential target for uptake by phagocytic cells and interaction with the immune system. Interaction of ENMs with phagocytes, lymphocytes and mast cells has induced a varied spectrum of undesired effects including inflammation, immunomodulation, and hypersensitivity. This interaction is mainly dependent on chemical and physical properties, including size and surface modification. ENM size has shown to induce the activation of the complement system. The considerable diversity of ENMs in size, composition, physicochemical properties, and surface modification has made it difficult to treat them as a single class of agents, resulting in a vast variety of toxicological findings from one ENM to another [235]. Mesoporous carbon nanomaterials (MCN), possessing a carbonaceous and mesoporous structure, have recently attracted enormous interests in clinical and pharmaceutical applications [236–241]. The unique properties of MCNs including high surface area, large pore volume, high drug capacity, and adjustable pore size have made them ideal for drug loading and controlled release in cancer therapy, suggesting the necessity to assess their immunotoxicity [236,239]. In 2018, Li et al. demonstrated the effects of unmodified and modified MCNs with polyvinylpyrrolidon (PVP) or polyethylene glycol (PEG) on dendritic cells (DCs), T lymphocytes and RAW264.7 macrophages in vitro. All studied MCNs in this work promoted DCs maturation and proliferation, however, MCN-PVP and MCNPEG significantly suppressed TNF-α and IL-6 secretion in these cells. Meanwhile, the modified MCNs induced apoptosis in T lymphocytes in a concentration-dependent manner. Taken together, these authors found that PVP- and PEG-MCN induced less immunogenicity as compared to pristine MCN [236]. In recent years, CNTs have been extensively investigated as drug nano-carries and diagnostic tools, raising concerns over their possible effects on the immune system [242]. CNTs, when injected intravenously, are capable of directly interacting with proteins and immune cells in blood and tissues [234]. Several reports have demonstrated that SWCNTs activate the expression of transcription factors including activator protein 1 (AP-1), tumor necrosis factor α (TNF-α) and nuclear factor kappa B (NF-κB) in macrophages, leading to T cell activation, release of proinflammatory cytokines, oxidative stress, necrosis, chromosomal aberrations, recruitment of leukocytes and induction of protective and anti-apoptotic gene expression [243–245]. Electron microscopic analysis has revealed that MWCNTs disrupt the integrity of the plasma membrane in macrophages via associating with its surface proteins like macrophage receptor with collagenous structure (MACRO) [246]. In a recent study, Zhang et al. investigated the pristine MWCNTs and PEG-modified MWCNTs immunotoxicity in BALB/c mice following intravenous administration. Exposure to pristine MWCNTs led to alterations in lymphocytes population in peripheral blood, increased IgM and IgG levels and decreased immune response to sheep erythrocytes. Morphological studies showed an inflammatory reaction in the injection site and mitochondria swelling in splenic macrophages. Further, they demonstrated that pristine nanotubes generated more immunotoxicity compared to PEG-MWCNTs. The histological analysis of this study revealed that pristine MWCNTs were extensively deposited in the tail vein of the injected site, leading to blackening of cells after 28 days. However, no obvious patholical changes were observed in the spleen and lymph nodes of the PEGMWCNT-administered mice. According to these authors, because of the enhanced solubility and dispersion of surface-modified CNTs, these CNMs were taken up by cells without reducing their viability [242].
5.6. Cardiotoxicity
Cardiotoxicity, the toxicity that affects the heart, comprises any direct effect of drugs on the heart or indirect heart damage through enhancement of haemodynamic flow or thrombotic events [247]. Recently, nano-diamonds (ND) as an allotrope of carbon have been reported to induce cardiotoxic effects in animals [248]. These nanoparticles have gained an increasing interest in various applications including biomedicine, biosensors, imaging probes, implants, lubricants and electrochemical coatings [249]. In vitro and in vivo studies have shown higher biocompatibility and lower toxicity of these nanoparticles as compared to other forms of carbon nanoparticle [248]. However, a recent study on the cardiotoxic effects of NDs in mice revealed that inhale exposure to NDs lead to a significant increase in ROS generation and lipid peroxidation, disruption of mitochondrial membrane potential (MMP), low levels of reduced glutathione (GSH) and high levels of glutathione disulfide (GSSG) in the heart tissue [249]. Meanwhile, studies on chicken embryo indicated that treatment with NDs and, to a lesser extent, graphite reduced vascularization and branched vessel's density via down-regulating pro-angiogenic basic fibroblast growth factor both at transcriptional and translational levels [248]. In spite of the cardiotoxic effects of some CNMs, fullerene has shown cardio-protective effects on doxorubicin-induced cardiotoxicity. Doxorubicin (DOX) is employed for the treatment of a wide variety of solid cancers, however, its cytotoxic effects and typically its impacts on heart and cell resistance limit its clinical application [250]. Several studies have shown that intra-peritoneum (IP) administration of fullerenol C60 (OH)24 (FNP) at doses of 50, 100 and 200 mg/kg, 30 min before administration of DOX (8 mg/kg) in rats prevented the DOX-induced reflex bradycardia and morphological changes like vacuolization of cardiomyocytes [251,252]. Graphene, a single layer of carbon atoms, has gained increasing interest in drug delivery, medical imaging techniques, tissue engineering, and solar cells. The release of graphene-based nanomaterials into aquatic environments may lead to serious cytotoxic effects in aquatic animals and, therefore, potential health risks in humans [253]. Recently, Manjunatha et al. demonstrated the cardiotoxicity, cardiovascular defects, retardation of cardiac looping and globin expression in zebrafish embryos. Their findings revealed that graphene exposure induces abnormal vascular growth, irregular branching of intersomatic vessels (ISVs) and formation of two-chambered heart structure [253]. GO, a water-soluble derivative of graphene, functionalized with ginsenoside Rh2 or basic amino acids (arginine (Arg) and lysine (Lys)) has been reported to decrease cardiac tissue destruction, compared to pristine GO. GO-Rh2, GO-Arg-Rh2, and GO-Lys-Rh2 have shown the lowest cardiotoxicity, which may arise from the larger size of the functionalized GO [254].
5.7. Genotoxicity and epigenetic toxicity
The processes that change DNA structure, information content and segregation, not inevitably associated with mutagenesis, are referred to as genotoxicity [255]. Fibrous CNMs would induce long-term genotoxic stress by direct interaction with DNA and mitotic apparatus or indirect interaction via inflammatory responses and oxidative stress. Nanotube's properties including charge density, functional groups, and surface area have shown to be critical in electrostatic interactions with DNA. For instance, cationic functionalized CNTs may lead to DNA condensation [256]. To date, various studies on CNTs genotoxicity have been published, demonstrating both genotoxic and non-genotoxic effects of these nanomaterials. This controversy may be due to different factors such as concentration, the route of exposure, duration of exposure and the dispersing agent in which the nanotube is solubilized [257]. Recently, Demir et al. demonstrated the effects of pristine MWCNTs, amidefunctionalized MWCNTs, and graphene on gene expression and mutagenicity in mouse lymphoma cell line. According to these authors, no mutagenic effects or significant changes in the expression of stress genes (Hsp70, Hsp80, CAT, SOD, and Ogg1) was observed at doses ≤250 μg/ml [257]. Furthermore, various reports have revealed that exposure to MWCNTs and SWCNTs may lead to aberrant epigenetic regulations like hyper- and hypo-methylation in the promoter region of specific genes in the lung or bronchial cells [155,258–260] and in Allium cepa root cells [261]. Epigenetics regulations are referred to as alterations in the expression of genes without changes in the primary sequence of DNA, leading to disease development [155]. Recently, Oner et al. demonstrated the differences between MWCNTs- and SWCNTsinduced DNA methylation alterations. Their findings revealed that MWCNTs-treatment led to hypomethylation of 2398 gene promoters, while, exposure to SWCNTs induced hyper- or hypo-methylation of 589 CpG sites. These alterations resulted in varied expression of genes, leading to changes in different cellular pathways, cell cycle, and DNA damage repair system. According to these authors, SWCNTs induced hyper-methylation in functionally important genes such as shroom family member 2 (SHROOM2), glutathione S-transferase pi 1 (GTSP1), SKI proto-oncogene (SKI) and both hypo- and hypermethylation of neurofibromatosis type I (NF1) gene. The studies of these investigators on nuclear deposition revealed that, in contrast to MWCNTs, a higher number of SWCNTs were deposited at cellular and nuclear levels, while, both CNTs showed physical contact with nuclei. It is also noteworthy that, no global methylation on 5-methylcytosine (5-mC) sites was detected following exposure to both types of CNTs [262]. CB is considered a poorly soluble and low toxicity particle, however, recent findings from a number of published studies have revealed that this particle may be directly genotoxic. The available studies suggest that CB does not directly interact with DNA, however, at high concentrations, this CNM, causes mutations in lung cells via inflammation and oxidative stress [49]. According to Modrzynska et al. studies, IT and IV exposure to CB led to long-lasting pulmonary inflammation, acute phase response (an orchestrated response to tissue injury [263]), infection or inflammation) and, subsequently, translocation of the nanoparticles from the lung to the liver. Their results demonstrated that IV injection of CB induced increased levels of DNA strand breaks in the liver after 1, 28 and 180 days, however, IT installation led to DNA strand breaks 28 and 180 days post-exposure. In contrast, oral administration of CB did not contribute to hepatic genotoxicity due to the lack of particle translocation, inflammation and acute phase response [264].
5.8. Dermatotoxicity
The skin's response to chemical substances, inducing damage, is referred to as dermatotoxicity. Penetration of chemicals to various layers of skin may result in different responses such as allergic contact dermatitis, irritation, contact urticaria, photosensitization and other cutaneous reactions [265]. Skin is the most probable route of exposure to nanomaterials, so the risks associated with their exposure and their underlying molecular mechanisms need to be well understood [163]. CNMs, when topically applied to the skin, initially have to penetrate the outermost layer of the epidermis, stratum corneum, consisting of several layers of keratinized dead cells embedded in an extracellular lipid matrix. This lipid pathway inhibits the penetration of substances and pathogens through the skin layers and, therefore, their access to the systemic circulation. However, in case of disease or occupational conditions that disrupt the stratum corneum layer, CNMs may penetrate these protective layers [266]. To date, only a few studies on the cytotoxic effects of CNMs on the skin have been published. In 2005, Ding et al. studied the cytotoxic mechanisms of MWCNTs and multi-walled carbon nano-onions (MWCNOs) in human skin fibroblast cells. According to these authors, the administration of MWCNTs and MWCNOs at cytotoxic doses promoted cell cycle arrest, apoptosis, and necrosis. Meanwhile, these CNMs perturbed multiple cellular pathways in association with the activation of genes involved in stress response, cell cycle regulation, cellular transport and metabolism [163]. In another study, Liao et al. determined the cytotoxicity of graphene and GO in skin fibroblasts by measuring mitochondrial activities in these cells. Their results revealed that the compacted graphene sheets, in contrast to less densely packed GO, induced more cytotoxicity in fibroblasts, generated more ROS and strongly associated with fibroblast's cell surface [116]. The cytotoxicity of graphene and GO has been also confirmed in human HaCaT skin keratinocytes. In this study, Pelin et al. reported the significant mitochondrial depolarization following 72 h of exposure to 100 μg/ml few-layer-graphene (FLG) and GO. This phenomenon seems to be mediated by ROS production that is likely induced by the activation of NADH dehydrogenase and xanthine oxidase as flavoprotein-based oxidative enzymes [267].
5.9. Carcinogenicity
Carcinogenicity is defined as the ability or tendency of a chemical to generate tumors, whether benign or malignant, increase their malignancy or accelerate tumor occurrence [268]. Various animal studies have reported that certain types of MWCNTs, similar to asbestos fibers, are carcinogenic to mesothelial cells and their pathogenic mechanism resembles that of asbestos. The carcinogenicity of these materials is mediated by various factors including length, rigidity, diameter and surface modification [269]. In contrast, the carcinogenicity of SWCNTs has not been reported yet. In 2016, Kasai et al. studied the carcinogenicity of MWCNT-7 in male and female rats following whole-body inhalation. According to these authors, exposure to 0.2 and 2 mg/L MWCNT-7 in male rats and 2 mg/L of this material in female rats, significantly increased bronchiolo-alveolar carcinoma, combined carcinomas, and adenomas. However, no pleural mesothelioma, cancer of the protective lining of the lung, was observed in the treated rats [270]. Recently, Sakamoto et al. conducted a comparative study to examine the carcinogenicity of 7 types of MWCNTs (M-CNT, N-CNT, WL-CNT, SD1-CNT, WS-CNT, SD2-CNT, and T-CNT), possessing different physic-chemical characteristics in Male Fischer rats. According to these authors, dehintraperitoneal injection of M-CNT, N-CNT, WL-CNT, and SD1-CNT (relatively longer than 5 μm and thicker than 60 nm) induced mesotheliomas with an absolute incidence of 100%. However, WS-CNT, SD2-CNT, and T-CNT with a curled and tightly tangled form and relatively small length and thickness (thinner than 50 nm) did not induce mesothelioma, except for SD2-and T-CNT with an absolute incidence rate of 1 per of 15 rats [271]. These findings are consistent with a previous study by Nagai et al.; demonstrating that MWCNTs with a relative diameter of 50 nm and high crystallinity promote mesotheliomagenecity in vivo. However, tangled (diameter ~ 2–20 nm) and thick (diameter ~ 150 nm) MWCNTs were less toxic, carcinogenic and inflammogenic [272]. According to the International Agency for Research on Cancer (IARC), epidemiological studies in the rubber industry and CB production provided inadequate evidence of carcinogenicity, however, the collected data from CB carcinogenicity in rodents provided sufficient evidence [273]. 5.10. The toxicity of magnetic carbon-based nanoparticles Toxicity of magnetic carbon-based nanoparticles have been assessed in some studies. Oh et al. designed a magnetic carbon nanoparticle with a controlled diameter for drug delivery. Polypyrrole (PPy) nanoparticles as the carbon precursor were fabricated via micelle templating technique and used for producing carbonized polypyrrole nanoparticles (CPyNs) through the carbonization process. For investigating cell viability, the CPyNs were incubated with human lung fibroblast cell line (IMR90) and ATP assay was done. After 24 h treatments with different diameters of CPyN (approximately 99, 76, 56 nm), the viability was decreased to ca. 72%, 63%, and 52%, respectively at the highest concentration of CPyNs (500 mgmL−1). Below 100 mgmL−1, no substantial decline was detected. It can be concluded that the cell viability of this magnetic carbon nanoparticle was depended to nanoparticle concentration and size [274]. Mu et al. investigated biocompatibility of Fe@CNPs by designing and synthesizing grafted Fe@CNPs with diverse chemistry modifications on surface. The effects of these nanoparticles on cellular processes containing cell uptake, oxidative stress, dynamic cellular responses, stimulation of cell apoptosis and cell cycle were measured. The obtained results indicate that the carboxylated Fe@CNPs with high concentration exhibited consistent toxicity. While other types of surface chemistry modifications present low toxicity and can be used in a wide range of biomedical applications [275]. In another study by Al Faraj et al., a novel nanocarrier based on magnetic SWCNTs was presented for cancer theranostics and sensitive detection using noninvasive MRI. For generating the homogeneous and stable dispersion of SWCNTs in water, polyvinylpyrrolidone (PVP) polymer was employed. In the next step, the SWCNTs were loaded with high iron oxide content. Then, the magnetic iron-tagged SWCNT complexes were labeled with a mouse Endoglin/CD105 monoclonal antibody for active and specific targeting of tumor sites in a 4T1-induced breast cancer murine model. To assess the in vitro biocompatibility of the magnetic iron-tagged SWCNT complexes, cell viability (MTT), mitochondrial membrane potential (JC-1) and ROS generation analysis were done. The obtained results of biocompatibility assays confirmed that the magnetic SWCNTs are safe for animal administration and can be used for monitoring cancers [276]. Hyperthermia is defined as a treatment aimed at raising the temperature of a region of the body to 40–43 °C [277,278]. This treatment method has been studied for over a century as a treatment for cancer and is almost always employed with other types of cancer therapy. Hyperthermia can lead to cell death by modulation of different cellular processes and also increase the anti-tumor effects of chemotherapy via enhancing the uptake of carcinostatics in tumor cells and preventing the repair of tumor cells [277,279]. In 2018, Zuo et al. fabricated magnetic CNTs with a low Curie temperature for self-regulating temperature hyperthermia [279]. In this research, the magnetic nanoparticles (Zn0.54 Co0.46 Cr0.6 Fe14O4) with Curie temperature at 45.7 °C were prepared. Subsequently, these magnetic nanoparticles were decorated on the surface of CNTs using the hydrothermal method; therefore, MCNTs with Curie temperature at 43 °C were produced. These MCNTs exhibit a self-regulating temperature at 42.7 °C under clinically applied magnetic field conditions. The in vitro cytotoxicity of MCNTs was quantitatively evaluated against Human epidermal keratinocyte (HaCaT) cell lines. The viability of HaCaT cells in the various concentrations of MCNTs (6.25 mg ml−1 to 100 mg ml−1) were reported above 85%, and there was no obvious toxicity effects on the MCNTstreated cell lines and control group (p-value > 0.05) [279].
6. Conclusion
The concept of Nanotechnology and its application in various fields of science and technology are growing at significant speed. Therefore, the chance of exposure to nanomaterials will continue to increase, this event will undoubtedly have both positive and negative effects on humans, animals, and the environment. The nanomaterial toxicity issue is one of significant and large concern in the world and as most of the researches in this area are not using the same methodologies and standards, the comparison is very hard. Among different types of nanomaterials, CNMs as of the main groups of nanomaterials does have extensive potential applications. But, toxicity and side effects of these materials have recently received significant attention. The obtained results of CNMs toxicity study indicate to the degree of toxicity is directly related to type, composition, shape, length, diameter, size and surface area. These parameters are mainly categorized with the type and shape of the CNMs that are extensively explained in this paper based on their toxicity routs including Neurotoxicity, Hepatotoxicity, Nephrotoxicity, Immunotoxicity, Cardiotoxicity, Genotoxicity and epigenetic toxicity, Dermatotoxicity, and Carcinogenicity. It can be concluded that the CNMs have very advantages in human life, but they also can have destructive effects on human health. Finally, we think the risk and toxicity assessments of CNMs and nanostructures are the starting points for developing the regulations to handle, produce and use them in industries and healthcare systems.
















Thorough, as usual. You might want to consider adding graphene hydroxide to the bunch. After all, 5G can convert graphene oxide into GH, the rapid killer, and revealing the truth about GH was what got Dr. Anreas Noack killed:
Dr. Andreas Noack, the most respected expert in graphene in Europe, was murdered (probably with a Directed Energy Weapon) at the end of November, 2021, only a few hours after he published his statement on the Internet in which he called the “covid” injections mass murder:
https://www.bitchute.com/video/WCfyNz0eK6zV/
https://forbiddenknowledgetv.net/murder-just-hours-after-publishing-the-secret-of-the-vaxx-dr-noack-is-dead/
https://www.henrymakow.com/2021/11/dr-andreas-noack-murdered.html
https://hipgnosis.co/2021/11/29/dr-noack-murdered-amid-proof-of-heart-inflammation-from-vax/
https://forbiddenknowledgetv.net/dr-noack-confirmed-dead-after-graphene-hydroxide-podcast/
https://report24.news/bestaetigt-dr-andreas-noack-verstarb-in-kaernten-oesterreich/
He had been arrested before during broadcasting a few weeks before by a team of heavily armed police:
https://insiderpaper.com/german-doctor-andreas-noack-arrest-video/
He knew what was coming, so he clearly sacrificed himself for the truth to come out.
The average person would never grasp this. I am not sure I do either. I am quite willing to make the assumption that ALL big pharma drugs, vaccines and mRNA substances are harmful to humans in some way, shape or form. Therefore, I avoid them as much as possible. They would be a last resort choice for me, if ever, including many OTC products. After all, they hide the true ingredients in all these products claiming that it is proprietary information. After the last 4 years, my trust factor has vanished forever.
So how do we know that we are not being systematically poisoned in plain sight? How do we know what the longer term risks really are? There are NO studies that confirm ANY drug is safe and effective for the longer term, especially when combined with other drugs and substances.