I think, it's important that people read through THAT, even for all those who don't want to buy it, I've copy it - because if you experiment with nano-wires in the brain, then everyone can imagine for themselves what comes out of it - of course everything is always made palatable to peoples with the "necessity" of health and security although the exact opposite is the case, but in the end it is a dangerous, inhuman and dehumanizing process, since nobody can know what the human being is being manipulated - you can already recognize in the first paragraph, first sentence
- with and how irrevocable the physiological and psychological damages can be and of course also will be!
Nanowire probes could drive high-resolution brain-machine interfaces
https://www.sciencedirect.com/science/article/abs/pii/S1748013219306929
Main text
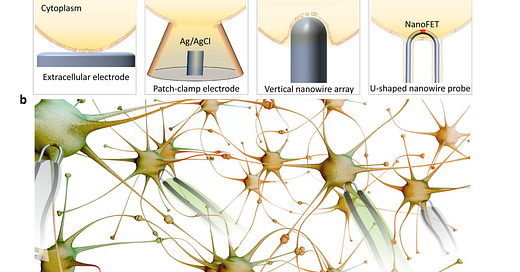
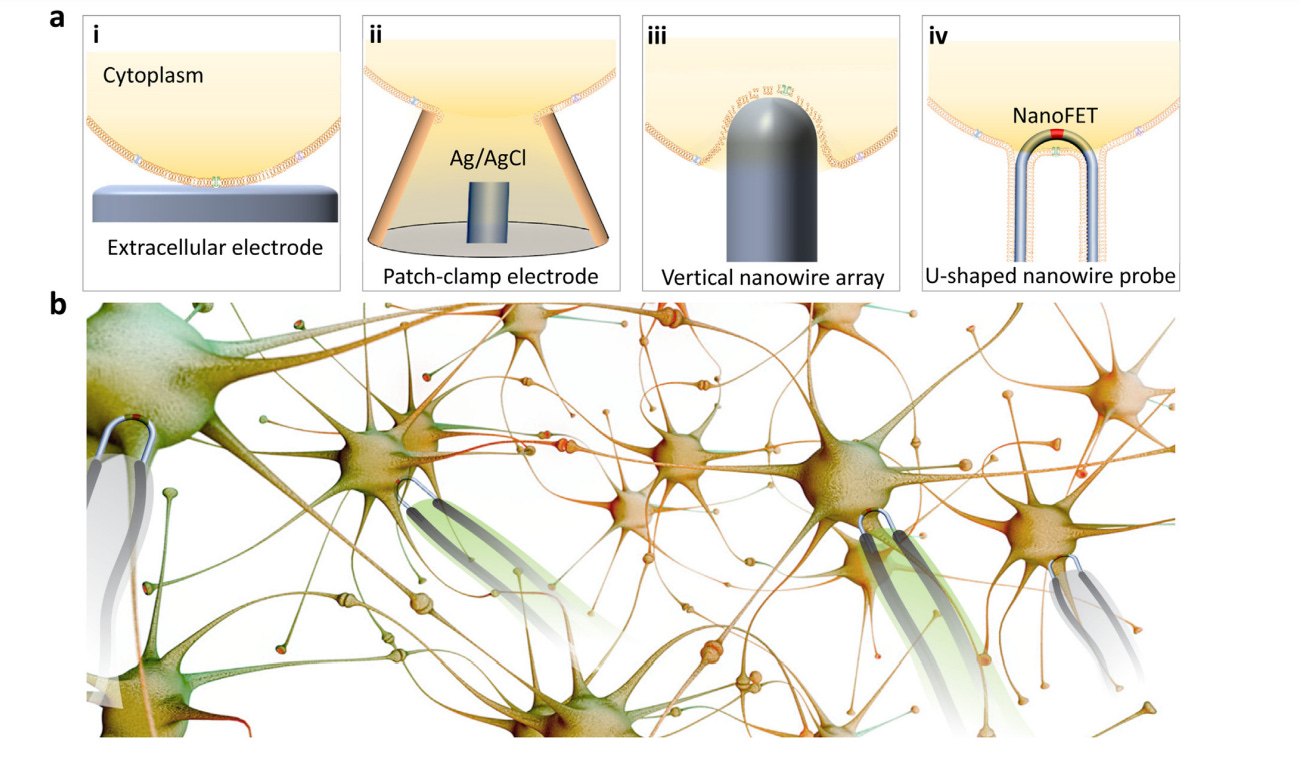
Brain-machine interfaces (BMIs) can serve as bidirectional connections that output electrical signals of brain activity or input electrical stimuli to modulate brain activity in concert with external machines, including computer processors and prosthetics, for human enhancement[1,2]. Reading electrical activity from neurons is the foundation of many BMI applications, such as brain mapping, that aims to understand brain functions by decoding the communication between neurons. Reading and processing this activity is also key to neural prosthetics in which brain activity is used to control devices such as artificial limbs. For these BMI applications, most of the in vivo recording tools used today read extracellular neural activity by detecting suprathreshold action potential signals that ‘leak’ outside of neurons (Fig. 1a (i)), while critical subthreshold events, such as synaptic potentials and dendritic integration, remain hidden [3]. To achieve the most information-rich readings, which could provide more detailed mapping of brain function and the finest control of neural prosthetics, electronic devices need to provide access to intracellular signals from multiple neurons comprising the neuronal circuits and networks of the brain [4].
The most widely used conventional method for intracellular recording is the patch-clamp electrode (Fig. 1a (ii)) [5], although it has limitations in terms of the probe tip size with respect to key subcellular elements such as synapses and ion channels, invasiveness and applicability to large-scale recording. Recent advances in nanowire-based bioelectronics [4], which can reach critical biological length scales of a few tens of nanometers, represent attractive candidates for next-generation cell electrophysiology tools that enable parallel and long-term detection of intracellular activity with high spatial resolution. The first work describing nanowire-enabled intracellular probes for electrogenic cells was achieved by the synthesis of nonlinear kinked nanowires, with a point-like field-effect transistor (FET) synthetically encoded at the tip [6]. Modification of the surfaces of the kinked nanowire FET probes with phospholipid bilayers yielded spontaneous insertion through the cell membrane of beating cardiomyocytes and subsequent intracellular action potential recording with an average peak amplitude of ∼80 mV as expected for these cells. To provide deterministic control over probe–cell interactions, kinked nanowire FETs have also been fabricated on the tip of free-standing probes that can be manipulated in 3D [7]. Significantly, simultaneous nanowire FET and patch-clamp recording from the same beating cardiomyocytes showed that the kinked nanowire probes recorded the same absolute intracellular signals as the standard of electrophysiology without any correction factors, thus opening up new capabilities in terms of spatial resolution and biomimetic cellular targeting. However, the kinked nanowire synthesis and assembly remains a challenge – they must be located and incorporated into the functional tools one-by-one, a process that is difficult to scale up.
Fig. 1. Nanowire-enabled intracellular recording. a, A comparison between (i) conventional extracellular electrodes, (ii) patch-clamp electrodes, (iii) vertical nanowire arrays, and (iv) ultrasmall U-shaped nanowire probes. Intracellular recording achieved by the ultrasmall U-shaped nanowire transistor probes has demonstrated minimal invasiveness and similar quality to those obtained with the patch-clamp electrodes. b, Nanowire probe arrays could enable network-level parallel recording in vitro or in vivo. - ‼️Note: And testing something "in vitro" or on the poor laboratory mice, as in reference (19), monkeys or ferrets has absolutely nothing to do with "in vivo" on humans, but the possibility to do it, then came in 2021!‼️
Vertical nanowire electrode arrays (Fig. 1a (iii)) have been developed as a scalable intracellular recording platform of electrogenic cells by several groups [8,9]. These nanowires typically have a height of a few micrometers, a diameter of tens to hundreds of nanometers. When cells are cultured on top of electrode arrays, the plasma membrane deforms and wraps around electrode, creating a tight junction at the interface [10]. Intracellular access can then be achieved by controlled membrane disruption, such as electroporation in which the application of voltage/current pulses at the nanowire tips transiently disrupt the local membrane patch [11,12]. Typically, the measured action potential amplitude is significantly increased immediately following electroporation, and gradually decreases over time as the membrane reseals. The top-down processes used to fabricate vertical nanowire arrays are intrinsically scalable and can be integrated with complementary metal-oxide–semiconductor (CMOS) circuits to enable parallel in vitro recording from thousands of connected cardiomyocytes and neurons [13,14]. Nevertheless, these studies also have limitations, including (i) the intracellular potentials are much lower than the signals expected for true intracellular recording, (ii) the signal-to-noise ratio depends on the electrochemical impedance of the fabricated metal nanowires, and (iii) the positions of the nanowires relative to the cultured cells cannot be manipulated. Additionally, the 2D substrate-based nanowire array platforms are not compatible with minimally-invasive 3D in vivo recording.
To address the limitations of synthesized kinked nanowire devices and fabricated vertical nanowire arrays, we have recently developed a scalable approach to U-shaped silicon nanowire arrays (Fig. 1a (iv)) [15]. The approach combines previous advances in deterministic shape-controlled nanowire transfer [16] with spatially defined semiconductor-to-metal transformation [17] to realize scalable nanowire FET probe arrays with controllable tip geometry and sensor size. Significantly, these new scalable probes enable parallel recording of intracellular action potentials from primary neurons. The silicon nanowires, with length-to-width ratio of more than 3000, are as flexible as cooked noodles. After bottom-up synthesis, they typically have disordered arrangement and orientations on the growth substrate. To incorporate them into functional device arrays that read intracellular electrical activities, we first patterned a silicon wafer with U-shaped polymer trenches, and then ‘comb’ the nanowires over the trenches [16]. In addition to removing tangles from the nanowire hair, the combing process forces the nanowires to conform to the designed U-shaped trenches, thus forming an array of ‘hairpin’-like U-shaped nanoscale devices. The center of each U-shaped nanowire, which points upward from the wafer surface, is defined as a small FET recorder by controlled semiconductor-to-metaltransformationofthenanowire arms [17], and can be inserted into the targeted neurons and cardiac cells for intracellular recording of signals up to or exceeding 100 mV which is comparable to the quality ofthose obtained with the patch-clamp electrodes. In addition, the cell-membrane mimicking phospholipid bilayer coating on the surfaces of the U-shaped nanowires allowed repeated insertion into multiple cells in parallel without causing damage.
During the development of the nanowire probes, we also compared many different curvatures of the U-shaped nanowire probe tips by varying systematically the top-down fabricated U-shaped trenches as well as different sizes of the FET devices. Importantly, this demonstrated a strong correlation between probes with smaller curvature/FET sizes, ease of internalization and larger recording amplitudes. These findings are consistent with the reported results demonstrating that the cell membrane curvature induced by tight interfacing with smaller nanostructures can induce activation of endocytosis and related biological pathways [18], indicating that using nanoscale topography to enhance device uptake is critical for developing tools that faithfully capture intracellular action potential features. Taking advantage of the parallel fabrication approach, we also explored different configurations of the device arrays: for example, having multiple nanowire devices on a single probe arm such that it is possible to read from multiple locations in a single cell, as well as ten of the probes that record signals from adjacent cells simultaneously to study signal propagation between cells. These modifications have the potential to make better tools for decoding the communication within and between complex neuron networks (Fig. 1b). The human brain contains about 86 billion neurons, and they are wired into complex circuits to process information conveyed by electrical signals. To study a largenumber ofneurons inthe brainvia intracellular recording, the conventional method of patch-clamp electrode is limited by its large micrometer tip size, and it can only read from a few neurons at a time. Recent advances in nanowire intracellular probes have achieved parallel fabrication of nanowire FET arrays, thereby addressing the long-standing challenge of scalable intracellular electrical recording with minimal invasiveness. Looking into the future, this deterministic nanowire-based fabrication strategy could be incorporated into other platforms [19–21], such as syringe-injectable mesh electronics [19], for multi-site 3D in vivo measurements. Two key challenges for the in vivo applications are the difficulty to achieve tight membrane/nanowire interfaces and long-term stability ofthe intracellular recording. We suggest that future studies exploring either (1) chemical anchoring via surface functionalization with groups that can bind to the actin cytoskeletal [22] or (2) physical anchoring using modulation of nanowire morphology [23] to increase the physical detachment force could improve the stability of the intracellular device configuration. Stable recording and interrogation of the same individual neurons intheneuronnetworkspromises tohelpdecipher complex neurological disorders [2], which develop over time, thus driving advanced BMIs with higher resolution, and perhaps eventually bringing ‼️‘cyborgs’‼️ to reality.
From the reference 19:
Syringe injectable electronics
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4591029/ - https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4591029/pdf/nihms-724760.pdf
References
[1] M.A. Lebedev, M.A. Nicolelis, Trends Neurosci. 29 (2006) 536.
[2] S.R. Patel, C.M. Lieber, Nat. Biotechnol. 37 (2019) 1007.
[3] D.L. Hunt, C. Lai, R.D. Smith, A.K. Lee, T.D. Harris, M. Barbic, Nat. Biomed. Eng. 3 (2019) 741.
[4] A. Zhang, C.M. Lieber, Chem. Rev. 116 (2016) 215.
[5] A. Molleman, Patch Clamping: An Introductory Guide to Patch Clamp Electrophysiology, John Wiley & Sons, Chichester, England, 2003.
[6] B. Tian, T. Cohen-Karni, Q. Qing, X. Duan, P. Xie, C.M. Lieber, Science 329 (2010) 830.
[7] Q. Qing, Z. Jiang, L. Xu, R. Gao, L. Mai, C.M. Lieber, Nat. Nanotechnol. 9 (2014) 142.
[8] J. Abbott, T. Ye, D. Ham, H. Park, Acc. Chem. Res. 51 (2018) 600.
[9] A.F. McGuire, F. Santoro, B. Cui, Annu. Rev. Anal. Chem. 11 (2018) 101.
[10] F. Santoro, W. Zhao, L.-M. Joubert, L. Duan, J. Schnitker, Y. van de Burgt, et al., ACS Nano 11 (2017) 8320.
[11] J.T. Robinson, M. Jorgolli, A.K. Shalek, M.-H. Yoon, R.S. Gertner, H. Park, Nat. Nanotechnol. 7 (2012) 180.
[12] C. Xie, Z. Lin, L. Hanson, Y. Cui, B. Cui, Nat. Nanotechnol. 7 (2012) 185.
[13] J. Abbott, T. Ye, L. Qin, M. Jorgolli, R.S. Gertner, D. Ham, et al., Nat. Nanotechnol. 12 (2017) 460.
[14] J. Abbott, T. Ye, K. Krenek, R.S. Gertner, S. Ban, Y. Kim, et al., Nat. Biomed. Eng. (2019), http://dx.doi.org/10.1038/s41551-019-0455-7.
[15] Y. Zhao, S.S. You, A. Zhang, J.-H. Lee, J. Huang, C.M. Lieber, Nat. Nanotechnol. 14 (2019) 783.
[16] Y. Zhao, J. Yao, L. Xu, M.N. Mankin, Y. Zhu, H. Wu, et al., Nano Lett. 16 (2016) 2644.
[17] Y. Wu, J. Xiang, C. Yang, W. Lu, C.M. Lieber, Nature 430 (2004) 61.
[18] H.-Y. Lou, W. Zhao, Y. Zeng, B. Cui, Acc. Chem. Res. 51 (2018) 1046.
[19] J. Liu, T.-M. Fu, Z. Cheng, G. Hong, T. Zhou, L. Jin, et al., Nat. Nanotechnol. 10 (2015) 629.
[20] L. Luan, X. Wei, Z. Zhao, J.J. Siegel, O. Potnis, C.A. Tuppen, et al.,Sci. Adv. 3 (2017), e1601966.
[21] S. Guan, J. Wang, X. Gu, Y. Zhao, R. Hou, H. Fan, et al., Sci. Adv. 5 (2019), eaav2842.
[22] J.-H. Lee, A. Zhang, S.S. You, C.M. Lieber, Nano Lett. 16 (2016) 1509.
[23] M. Dipalo, A.F. McGuire, H.-Y. Lou, V. Caprettini, G. Melle, G. Bruno, et al., Nano Lett. 18 (2018) 6100.
And in my opinion, one can now also imagine why Charles Lieber and the two others were arrested in January 2020, when the WHO (Thedros), Fauci, Collins etc. and their backers and henchmen had decided or had been the "order" to put this crime against humanity into practice, because Lieber and the other two could have immediately explained what was actually being researched in the so-called "bio-laboratory" in "Wuhan" (nanotechnology, toxic-chemical substances, structures and forms of all kinds, but NO "virus"! ) and is being researched (and as you can read here,
the Air Force Office of Scientific Research also knew about it, as it supported this "research"), and not how they all are still trying to tell humanity this fairy tale of a non-existent "laboratory virus",(which would be absolutely impossible!), in order to continue to maintain a conscious lie that everyone of them knew and knows!!
A rogue who'd think of something bad about it, right?🤔…😉
❤️❤️❤️





Great job.
Again, the old adage seems to be at work:
"If something can be done, someone will do it."
Apparently, "someone" is already doing it.